Autoantibodies in neurological disease
- PMID: 33976421
- PMCID: PMC8111372
- DOI: 10.1038/s41577-021-00543-w
Autoantibodies in neurological disease
Abstract
The realization that autoantibodies can contribute to dysfunction of the brain has brought about a paradigm shift in neurological diseases over the past decade, offering up important novel diagnostic and therapeutic opportunities. Detection of specific autoantibodies to neuronal or glial targets has resulted in a better understanding of central nervous system autoimmunity and in the reclassification of some diseases previously thought to result from infectious, 'idiopathic' or psychogenic causes. The most prominent examples, such as aquaporin 4 autoantibodies in neuromyelitis optica or NMDAR autoantibodies in encephalitis, have stimulated an entire field of clinical and experimental studies on disease mechanisms and immunological abnormalities. Also, these findings inspired the search for additional autoantibodies, which has been very successful to date and has not yet reached its peak. This Review summarizes this rapid development at a point in time where preclinical studies have started delivering fundamental new data for mechanistic understanding, where new technologies are being introduced into this field, and - most importantly - where the first specifically tailored immunotherapeutic approaches are emerging.
© 2021. Springer Nature Limited.
Conflict of interest statement
The author declares no competing interests.
Figures

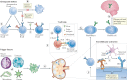
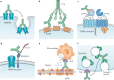
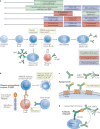
References
-
- Pollak TA, et al. Autoimmune psychosis: an international consensus on an approach to the diagnosis and management of psychosis of suspected autoimmune origin. Lancet Psychiatry. 2019;7:93–108. - PubMed
-
- Graus F, Dalmau J. Paraneoplastic neurological syndromes in the era of immune-checkpoint inhibitors. Nat. Rev. Clin. Oncol. 2019;16:535–548. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

