Thymoquinone: A Promising Natural Compound with Potential Benefits for COVID-19 Prevention and Cure
- PMID: 33976534
- PMCID: PMC8106451
- DOI: 10.2147/DDDT.S308863
Thymoquinone: A Promising Natural Compound with Potential Benefits for COVID-19 Prevention and Cure
Abstract
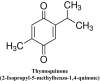
COVID-19 has caused a major global health crisis, as excessive inflammation, oxidation, and exaggerated immune response in some sufferers can lead to a condition known as cytokine storm, which may progress to acute respiratory distress syndrome (ARDs), which can be fatal. So far, few effective drugs have emerged to assist in the treatment of patients with COVID-19, though some herbal medicine candidates may assist in the fight against COVID-19 deaths. Thymoquinone (TQ), the main active ingredient of black seed oil, possesses antioxidant, anti-inflammatory, antiviral, antimicrobial, immunomodulatory and anticoagulant activities. TQ also increases the activity and number of cytokine suppressors, lymphocytes, natural killer cells, and macrophages, and it has demonstrated antiviral potential against a number of viruses, including murine cytomegalovirus, Epstein-Barr virus, hepatitis C virus, human immunodeficiency virus, and other coronaviruses. Recently, TQ has demonstrated notable antiviral activity against a SARSCoV-2 strain isolated from Egyptian patients and, interestingly, molecular docking studies have also shown that TQ could potentially inhibit COVID-19 development through binding to the receptor-binding domain on the spike and envelope proteins of SARS-CoV-2, which may hinder virus entry into the host cell and inhibit its ion channel and pore forming activity. Other studies have shown that TQ may have an inhibitory effect on SARS CoV2 proteases, which could diminish viral replication, and it has also demonstrated good antagonism to angiotensin-converting enzyme 2 receptors, allowing it to interfere with virus uptake into the host cell. Several studies have also noted its potential protective capability against numerous chronic diseases and conditions, including diabetes, hypertension, dyslipidemia, asthma, renal dysfunction and malignancy. TQ has recently been tested in clinical trials for the treatment of several different diseases, and this review thus aims to highlight the potential therapeutic effects of TQ in the context of the COVID-19 pandemic.
Keywords: COVID-19; natural; therapeutic benefits; thymoquinone.
© 2021 Badary et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous