Real-Time Monitoring and Step-by-Step Guidance for Transapical Mitral Valve Edge-to-Edge Repair Using Transesophageal Echocardiography
- PMID: 33976590
- PMCID: PMC8084671
- DOI: 10.1155/2021/6659261
Real-Time Monitoring and Step-by-Step Guidance for Transapical Mitral Valve Edge-to-Edge Repair Using Transesophageal Echocardiography
Abstract
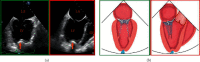
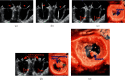
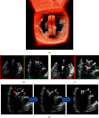
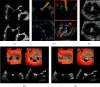
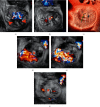
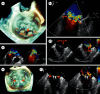
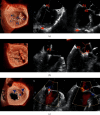
MitraClip edge-to-edge (E2E) repair system is the only transcatheter device recommended in the current guidelines for treating mitral regurgitation (MR). The percutaneous femoral venous transseptal access of MitraClip requires a complex steerable delivery system and may thus be technically complex to optimally position and deploy the clip onto the mitral valve. A transapical approach for E2E repair has been devised to treat MR for the ease of operation (ValveClamp system, Hanyu Medical Technology, Shanghai). The first-in-human study of ValveClamp has demonstrated its early feasibility and effectiveness for the treatment of patients with degenerative MR. Transesophageal echocardiography (TEE) is the only imaging modality required for intraoperative guidance of ValveClamp implantation. Successful implantation depends on accurate localization and orientation of the clamp and efficient intraoperative communication between the echocardiographer and the intervention team. Thus, the focus of this review is on elaborating how two-dimensional (2D) and three-dimensional (3D) TEE are used in clinical practice to guide ValveClamp implantation and it may facilitate the understanding of simplicity and safety of this novel procedure. We also describe the implementation of several novel advancements in 3D TEE imaging, which improve the confidence of image interpretation for intraoperative guidance and expedite implantation times.
Copyright © 2021 Zhenyi Ge et al.
Conflict of interest statement
Dr. Daxin Zhou and Dr. Wenzhi Pan are consultants for Hanyu Medical Technology. All the other authors have reported that they have no relationships relevant to the content of this paper to disclose.
Figures

References
-
- Geyer M., Sotiriou E., Tamm A. R., et al. Advanced protocol for three-dimensional transesophageal echocardiography guidance implementing real-time multiplanar reconstruction for transcatheter mitral valve repair by direct annuloplasty. Journal of the American Society of Echocardiography. 2019;32(10):1359–1365. doi: 10.1016/j.echo.2019.05.015. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
