Monitoring cell productivity for the production of recombinant proteins by flow cytometry: An effective application using the cold capture assay
- PMID: 33976601
- PMCID: PMC8092981
- DOI: 10.1002/elsc.202000049
Monitoring cell productivity for the production of recombinant proteins by flow cytometry: An effective application using the cold capture assay
Abstract
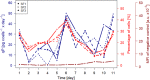
Due to the increasing economic and social relevance of biotherapeutics, their production processes are continually being reconsidered and reoptimized in an effort to secure higher product concentrations and qualities. Monitoring the productivity of cultured cells is therefore a critically important part of the cultivation process. Traditionally, this is achieved by determining the overall product titer by high performance liquid chromatography (HPLC), and then calculating the specific cell productivity based on this titer and an associated viable cell density. Unfortunately, this process is typically time-consuming and laborious. In this study, the productivity of Chinese Hamster Ovary (CHO) cells expressing a monoclonal antibody was analyzed over the course of the cultivation process. In addition to calculating the specific cell productivity based on the traditional product titer determined by HPLC analysis, culture productivity of single cells was also analyzed via flow cytometry using a cold capture assay. The cold capture assay is a cell surface labelling technique described by Brezinsky et al., which allows for the visualization of a product on the surface of the producing cell. The cell productivity results obtained via HPLC and the results of cold capture assay remained in great accordance over the whole cultivation process. Accordingly, our study demonstrates that the cold capture assay offers an interesting, comparatively time-effective, and potentially cheaper alternative for monitoring the productivity of a cell culture.
Keywords: CHO cells; mammalian cell culture; monoclonal antibody production; secretion assay; specific cell productivity.
© 2021 The Authors. Engineering in Life Sciences published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors have declared no conflict of interest.
Figures

References
-
- Brezinsky, S. C. G. , Chiang, G. G. , Szilvasi, A. , Mohan, S. , et al., A simple method for enriching populations of transfected CHO cells for cells of higher specific productivity. J. Immunol. Methods 2003, 277, 141–155. - PubMed
-
- Pichler, J. , Galosy, S. , Mott, J. , and Borth, N. , Selection of CHO host cell subclones with increased specific antibody production rates by repeated cycles of transient transfection and cell sorting. Biotechnol. Bioeng. 2011, 108, 386–394. - PubMed
-
- Kumar, N. and Borth, N. , Flow‐cytometry and cell sorting: an efficient approach to investigate productivity and cell physiology in mammalian cell factories. Methods 2012, 56, 366–374. - PubMed
-
- Pichler, J. , Hesse, F. , Wieser, M. , Kunert, R. , et al., A study on the temperature dependency and time course of the cold capture antibody secretion assay. J. Biotechnol. 2009, 141, 80–83. - PubMed
-
- Fischer, S. , Handrick, R. , and Otte, K. , The art of CHO cell engineering: a comprehensive retrospect and future perspectives. Biotechnol. Adv. 2015, 33, 1878–1896. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
