Safety assessment of titanium dioxide (E171) as a food additive
- PMID: 33976718
- PMCID: PMC8101360
- DOI: 10.2903/j.efsa.2021.6585
Safety assessment of titanium dioxide (E171) as a food additive
Abstract
The present opinion deals with an updated safety assessment of the food additive titanium dioxide (E 171) based on new relevant scientific evidence considered by the Panel to be reliable, including data obtained with TiO2 nanoparticles (NPs) and data from an extended one-generation reproductive toxicity (EOGRT) study. Less than 50% of constituent particles by number in E 171 have a minimum external dimension < 100 nm. In addition, the Panel noted that constituent particles < 30 nm amounted to less than 1% of particles by number. The Panel therefore considered that studies with TiO2 NPs < 30 nm were of limited relevance to the safety assessment of E 171. The Panel concluded that although gastrointestinal absorption of TiO2 particles is low, they may accumulate in the body. Studies on general and organ toxicity did not indicate adverse effects with either E 171 up to a dose of 1,000 mg/kg body weight (bw) per day or with TiO2 NPs (> 30 nm) up to the highest dose tested of 100 mg/kg bw per day. No effects on reproductive and developmental toxicity were observed up to a dose of 1,000 mg E 171/kg bw per day, the highest dose tested in the EOGRT study. However, observations of potential immunotoxicity and inflammation with E 171 and potential neurotoxicity with TiO2 NPs, together with the potential induction of aberrant crypt foci with E 171, may indicate adverse effects. With respect to genotoxicity, the Panel concluded that TiO2 particles have the potential to induce DNA strand breaks and chromosomal damage, but not gene mutations. No clear correlation was observed between the physico-chemical properties of TiO2 particles and the outcome of either in vitro or in vivo genotoxicity assays. A concern for genotoxicity of TiO2 particles that may be present in E 171 could therefore not be ruled out. Several modes of action for the genotoxicity may operate in parallel and the relative contributions of different molecular mechanisms elicited by TiO2 particles are not known. There was uncertainty as to whether a threshold mode of action could be assumed. In addition, a cut-off value for TiO2 particle size with respect to genotoxicity could not be identified. No appropriately designed study was available to investigate the potential carcinogenic effects of TiO2 NPs. Based on all the evidence available, a concern for genotoxicity could not be ruled out, and given the many uncertainties, the Panel concluded that E 171 can no longer be considered as safe when used as a food additive.
Keywords: CAS No 13463‐67-7; E 171; Titanium dioxide.
© 2021 European Food Safety Authority. EFSA Journal published by John Wiley and Sons Ltd on behalf of European Food Safety Authority.
Figures

*: Some of the studies used more than one test material and in these cases, the results are reported separately.
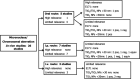

*: Some of the studies used more than one test material and in these cases, the results are reported separately.
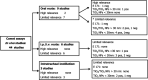
*: Some of the studies used more than one test material and, in these cases, the results are reported separately.

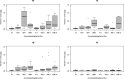
References
-
- Akera T and Brody TM, 1978. The role of Na+, K+‐ATPase in the inotropic action of digitalis. Pharmacological Reviews, 29, 187–220. - PubMed
-
- Ali K, Abul QF, Dwivedi S, Abdel‐Salam EM, Ansari SM, Saquib Q, Faisal M, Al‐Khedhairy AA, Al‐Shaeri M and Musarrat J, 2018. Titanium dioxide nanoparticles preferentially bind in subdomains IB, IIA of HSA and minor groove of DNA. Journal of Biomolecular Structure and Dynamics, 36, 2530–2542. - PubMed
-
- Ali SA, Rizk MZ, Hamed MA, Aboul‐Ela EI, El‐Rigal NS, Aly HF and Abdel‐Hamid AZ, 2019. Assessment of titanium dioxide nanoparticles toxicity via oral exposure in mice: effect of dose and particle size. Biomarkers: biochemical indicators of exposure, response, and susceptibility to chemicals, 24, 492–498. - PubMed
-
- Alsudir S and Lai EPC, 2017. Electrosteric stabilization of colloidal TiO2 nanoparticles with DNA and polyethylene glycol for selective enhancement of UV detection sensitivity in capillary electrophoresis analysis. Analytical and Bioanalytical Chemistry, 409. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
