MgFe-LDH Nanoparticles: A Promising Leukemia Inhibitory Factor Replacement for Self-Renewal and Pluripotency Maintenance in Cultured Mouse Embryonic Stem Cells
- PMID: 33977050
- PMCID: PMC8097378
- DOI: 10.1002/advs.202003535
MgFe-LDH Nanoparticles: A Promising Leukemia Inhibitory Factor Replacement for Self-Renewal and Pluripotency Maintenance in Cultured Mouse Embryonic Stem Cells
Abstract
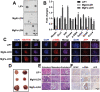
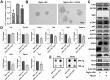
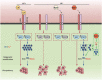
Leukemia inhibitory factor (LIF), an indispensable bioactive protein that sustains self-renewal and pluripotency in stem cells, is vital for mouse embryonic stem cell (mESC) culture. Extensive research is conducted on reliable alternatives for LIF as its clinical application in stable culture and large-scale expansion of ESCs is limited by its instability and high cost. However, few studies have sought to replace LIF with nanoparticles to provide a xeno-free culture condition. MgAl-LDH (layered double hydroxide) nanoparticles can partially replace LIF in maintaining pluripotency of mESCs; however, the requirement and tolerance for aluminum ions in mice are far lesser than those of iron ions. Hence, MgFe-LDH nanoparticles are selected for this study. MgFe-LDH is superior to MgAl-LDH in maintaining self-renewal and pluripotency of mESCs, in the absence of LIF and mouse embryonic fibroblast. Furthermore, combined transcriptomic and proteomic analysis confirms that MgFe-LDH can activate the LIF receptor (LIFR)/phosphatidylinositol 3-kinase (PI3K)/protein kinase B(AKT), LIFR/JAK/janus kinase (JAK)/signal transducer and activator of transcription 3(STAT3), and phospho-signal transducer and activator of transcription 3(p-STAT3)/ten-eleven translocation (TET) signaling pathways, while the extra Fe2+ provided by MgFe-LDH would also enhance TET1/2 abundance thus affecting the TET1/2 regulated pluripotency related marker expression and TET1/2 meditated DNA demethylation. These results suggest that MgFe-LDH nanoparticles can thus be used as an affordable and efficient replacement for LIF in mESC cultivation.
Keywords: LIFR/JAK/STAT3; MgFe‐LDH nanoparticles; combined transcriptomic and proteomic analysis; embryonic stem cells; pluripotency.
© 2021 The Authors. Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Wang C., Yue H. B., Feng Q., Xu B. Z., Bian L. M., Shi P., ACS Appl. Mater. Interfaces 2018, 10, 29299. - PubMed
-
- Chong J. J. H., Yang X. L., Don C. W., Minami E., Liu Y. W., Weyers J. J., Mahoney W. M., Van Biber B., Cook S. M., Palpant N. J., Gantz J. A., Fugate J. A., Muskheli V., Gough G. M., Vogel K. W., Astley C. A., Hotchkiss C. E., Baldessari A., Pabon L., Reinecke H., Gill E. A., Nelson V., Kiem H. P., Laflamme M. A., Murry C. E., Nature 2014, 510, 273. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
