Rethinking CRITID Procedure of Brain Targeting Drug Delivery: Circulation, Blood Brain Barrier Recognition, Intracellular Transport, Diseased Cell Targeting, Internalization, and Drug Release
- PMID: 33977060
- PMCID: PMC8097396
- DOI: 10.1002/advs.202004025
Rethinking CRITID Procedure of Brain Targeting Drug Delivery: Circulation, Blood Brain Barrier Recognition, Intracellular Transport, Diseased Cell Targeting, Internalization, and Drug Release
Abstract
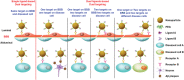
The past decades have witnessed great progress in nanoparticle (NP)-based brain-targeting drug delivery systems, while their therapeutic potentials are yet to be fully exploited given that the majority of them are lost during the delivery process. Rational design of brain-targeting drug delivery systems requires a deep understanding of the entire delivery process along with the issues that they may encounter. Herein, this review first analyzes the typical delivery process of a systemically administrated NPs-based brain-targeting drug delivery system and proposes a six-step CRITID delivery cascade: circulation in systemic blood, recognizing receptor on blood-brain barrier (BBB), intracellular transport, diseased cell targeting after entering into parenchyma, internalization by diseased cells, and finally intracellular drug release. By dissecting the entire delivery process into six steps, this review seeks to provide a deep understanding of the issues that may restrict the delivery efficiency of brain-targeting drug delivery systems as well as the specific requirements that may guarantee minimal loss at each step. Currently developed strategies used for troubleshooting these issues are reviewed and some state-of-the-art design features meeting these requirements are highlighted. The CRITID delivery cascade can serve as a guideline for designing more efficient and specific brain-targeting drug delivery systems.
Keywords: brain‐targeting; drug delivery; dual‐targeting; intracellular trafficking; stimulus‐responsive.
© 2021 The Authors. Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures










Similar articles
-
Perspectives on Dual Targeting Delivery Systems for Brain Tumors.J Neuroimmune Pharmacol. 2017 Mar;12(1):6-16. doi: 10.1007/s11481-016-9687-4. Epub 2016 Jun 8. J Neuroimmune Pharmacol. 2017. PMID: 27270720 Review.
-
Recent expansions of novel strategies towards the drug targeting into the brain.Int J Nanomedicine. 2019 Jul 30;14:5895-5909. doi: 10.2147/IJN.S210876. eCollection 2019. Int J Nanomedicine. 2019. PMID: 31440051 Free PMC article. Review.
-
Strategies to improve drug delivery across the blood-brain barrier.Clin Pharmacokinet. 2007;46(7):553-76. doi: 10.2165/00003088-200746070-00002. Clin Pharmacokinet. 2007. PMID: 17596102 Review.
-
Nanoparticle technology for drug delivery across the blood-brain barrier.Drug Dev Ind Pharm. 2002 Jan;28(1):1-13. doi: 10.1081/ddc-120001481. Drug Dev Ind Pharm. 2002. PMID: 11858519 Review.
-
Central nervous system delivery of molecules across the blood-brain barrier.Neurochem Int. 2021 Mar;144:104952. doi: 10.1016/j.neuint.2020.104952. Epub 2021 Jan 2. Neurochem Int. 2021. PMID: 33400964 Review.
Cited by
-
Engineered nanoparticles for precise targeted drug delivery and enhanced therapeutic efficacy in cancer immunotherapy.Acta Pharm Sin B. 2024 Aug;14(8):3432-3456. doi: 10.1016/j.apsb.2024.05.010. Epub 2024 May 13. Acta Pharm Sin B. 2024. PMID: 39220871 Free PMC article. Review.
-
Recent Progress of RGD Modified Liposomes as Multistage Rocket Against Cancer.Front Pharmacol. 2022 Jan 25;12:803304. doi: 10.3389/fphar.2021.803304. eCollection 2021. Front Pharmacol. 2022. PMID: 35145405 Free PMC article. Review.
-
Posterity of nanoscience as lipid nanosystems for Alzheimer's disease regression.Mater Today Bio. 2023 Jun 17;21:100701. doi: 10.1016/j.mtbio.2023.100701. eCollection 2023 Aug. Mater Today Bio. 2023. PMID: 37415846 Free PMC article. Review.
-
Nano-Encapsulated Taro Lectin Can Cross an in vitro Blood-Brain Barrier, Induce Apoptosis and Autophagy and Inhibit the Migration of Human U-87 MG Glioblastoma Cells.Int J Nanomedicine. 2025 Apr 29;20:5573-5591. doi: 10.2147/IJN.S511506. eCollection 2025. Int J Nanomedicine. 2025. PMID: 40321803 Free PMC article.
-
Non-Viral Carriers for Nucleic Acids Delivery: Fundamentals and Current Applications.Life (Basel). 2023 Mar 29;13(4):903. doi: 10.3390/life13040903. Life (Basel). 2023. PMID: 37109432 Free PMC article. Review.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
