DNA-Grafted Hyaluronic Acid System with Enhanced Injectability and Biostability for Photo-Controlled Osteoarthritis Gene Therapy
- PMID: 33977074
- PMCID: PMC8097319
- DOI: 10.1002/advs.202004793
DNA-Grafted Hyaluronic Acid System with Enhanced Injectability and Biostability for Photo-Controlled Osteoarthritis Gene Therapy
Abstract
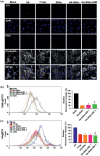
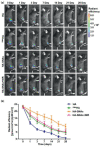
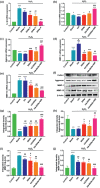
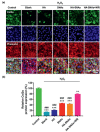
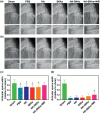
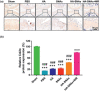
Gene therapy is identified as a powerful strategy to overcome the limitations of traditional therapeutics to achieve satisfactory effects. However, various challenges related to the dosage form, delivery method, and, especially, application value, hampered the clinical transition of gene therapy. Here, aiming to regulate the cartilage inflammation and degeneration related abnormal IL-1β mRNA expression in osteoarthritis (OA), the interference oligonucleotides is integrated with the Au nanorods to fabricate the spherical nucleic acids (SNAs), to promote the stability and cell internalization efficiency. Furthermore, the complementary oligonucleotides are grafted onto hyaluronic acid (HA) to obtained DNA-grafted HA (DNAHA) for SNAs delivery by base pairing, resulting in significantly improved injectability and bio-stability of the system. After loading SNAs, the constructed DNAHA-SNAs system (HA-SNAs) performs a reversible NIR-triggered on-demand release of SNAs by photo-thermal induced DNA dehybridization and followed by post-NIR in situ hybridization. The in vitro and in vivo experiments showed that this system down-regulated catabolic proteases and up-regulated anabolic components in cartilage over extended periods of time, to safeguard the chondrocytes against degenerative changes and impede the continual advancement of OA.
Keywords: DNA grafted hyaluronic acid; anti‐inflammation; enhanced injectability; long‐term bio‐stability; osteoarthritis gene therapy; spherical nucleic acids.
© 2021 The Authors. Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
