Nanomedicine for acute respiratory distress syndrome: The latest application, targeting strategy, and rational design
- PMID: 33977080
- PMCID: PMC8102084
- DOI: 10.1016/j.apsb.2021.04.023
Nanomedicine for acute respiratory distress syndrome: The latest application, targeting strategy, and rational design
Abstract
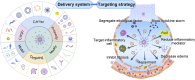
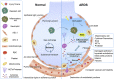
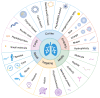
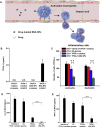
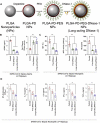
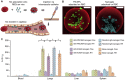
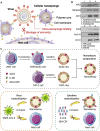
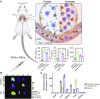
Acute respiratory distress syndrome (ARDS) is characterized by the severe inflammation and destruction of the lung air-blood barrier, leading to irreversible and substantial respiratory function damage. Patients with coronavirus disease 2019 (COVID-19) have been encountered with a high risk of ARDS, underscoring the urgency for exploiting effective therapy. However, proper medications for ARDS are still lacking due to poor pharmacokinetics, non-specific side effects, inability to surmount pulmonary barrier, and inadequate management of heterogeneity. The increased lung permeability in the pathological environment of ARDS may contribute to nanoparticle-mediated passive targeting delivery. Nanomedicine has demonstrated unique advantages in solving the dilemma of ARDS drug therapy, which can address the shortcomings and limitations of traditional anti-inflammatory or antioxidant drug treatment. Through passive, active, or physicochemical targeting, nanocarriers can interact with lung epithelium/endothelium and inflammatory cells to reverse abnormal changes and restore homeostasis of the pulmonary environment, thereby showing good therapeutic activity and reduced toxicity. This article reviews the latest applications of nanomedicine in pre-clinical ARDS therapy, highlights the strategies for targeted treatment of lung inflammation, presents the innovative drug delivery systems, and provides inspiration for strengthening the therapeutic effect of nanomedicine-based treatment.
Keywords: ACE2, angiotensin-converting enzyme 2; AEC II, alveolar type II epithelial cells; AM, alveolar macrophages; ARDS, acute respiratory distress syndrome; Acute lung injury; Acute respiratory distress syndrome; Anti-inflammatory therapy; BALF, bronchoalveolar lavage fluid; BSA, bovine serum albumin; CD, cyclodextrin; CLP, cecal ligation and perforation; COVID-19; COVID-19, coronavirus disease 2019; DOPE, phosphatidylethanolamine; DOTAP, 1-diolefin-3-trimethylaminopropane; DOX, doxorubicin; DPPC, dipalmitoylphosphatidylcholine; Drug delivery; ECM, extracellular matrix; ELVIS, extravasation through leaky vasculature and subsequent inflammatory cell-mediated sequestration; EPCs, endothelial progenitor cells; EPR, enhanced permeability and retention; EVs, extracellular vesicles; EphA2, ephrin type-A receptor 2; Esbp, E-selectin-binding peptide; FcgR, Fcγ receptor; GNP, peptide-gold nanoparticle; H2O2, hydrogen peroxide; HO-1, heme oxygenase-1; ICAM-1, intercellular adhesion molecule-1; IKK, IκB kinase; IL, interleukin; LPS, lipopolysaccharide; MERS, Middle East respiratory syndrome; MPMVECs, mouse pulmonary microvascular endothelial cells; MPO, myeloperoxidase; MSC, mesenchymal stem cells; NAC, N-acetylcysteine; NE, neutrophil elastase; NETs, neutrophil extracellular traps; NF-κB, nuclear factor-κB; Nanomedicine; PC, phosphatidylcholine; PCB, poly(carboxybetaine); PDA, polydopamine; PDE4, phosphodiesterase 4; PECAM-1, platelet-endothelial cell adhesion molecule; PEG, poly(ethylene glycol); PEI, polyetherimide; PEVs, platelet-derived extracellular vesicles; PLGA, poly(lactic-co-glycolic acid); PS-PEG, poly(styrene-b-ethylene glycol); Pathophysiologic feature; RBC, red blood cells; RBD, receptor-binding domains; ROS, reactive oxygen species; S1PLyase, sphingosine-1-phosphate lyase; SARS, severe acute respiratory syndrome; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SDC1, syndecan-1; SORT, selective organ targeting; SP, surfactant protein; Se, selenium; Siglec, sialic acid-binding immunoglobulin-like lectin; TLR, toll-like receptor; TNF-α, tumor necrosis factor-α; TPP, triphenylphosphonium cation; Targeting strategy; YSA, YSAYPDSVPMMS; cRGD, cyclic arginine glycine-d-aspartic acid; iNOS, inducible nitric oxide synthase; rSPANb, anti-rat SP-A nanobody; scFv, single chain variable fragments.
© 2021 Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences. Production and hosting by Elsevier B.V.
Figures



References
-
- Fan E., Brodie D., Slutsky A.S. Acute respiratory distress syndrome: advances in diagnosis and treatment. J Am Med Assoc. 2018;319:698–710. - PubMed
-
- Bellani G., Laffey J.G., Pham T., Fan E., Brochard L., Esteban A. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 Countries. J Am Med Assoc. 2016;315:788–800. - PubMed
-
- Thompson B.T., Chambers R.C., Liu K.D. Acute respiratory distress syndrome. N Engl J Med. 2017;377:562–572. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

