Emerging roles for the autophagy machinery in extracellular vesicle biogenesis and secretion
- PMID: 33977236
- PMCID: PMC8103724
- DOI: 10.1096/fba.2020-00138
Emerging roles for the autophagy machinery in extracellular vesicle biogenesis and secretion
Abstract
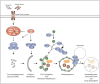
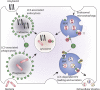
Autophagy classically functions to maintain cell health during stressful conditions by targeting cytosolic components for degradation and recycling via lysosomal pathways. However, accumulating evidence also supports roles for autophagy-related genes (ATGs) in non-degradative processes including cellular secretion. Here, we review emerging roles for the autophagy machinery in regulating extracellular vesicle loading and secretion and discuss how functional coupling of these pathways may impact normal physiology and disease.
© 2021 The Authors. FASEB BioAdvances published by the Federation of American Societies for Experimental Biology.
Conflict of interest statement
JD is the member of the Scientific Advisory Board of Vescor Therapeutics, LLC.
Figures
References
-
- van Niel G, D'Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol. 2018;19:213‐228. - PubMed
-
- Pegtel DM, Gould SJ. Exosomes. Annu Rev Biochem. 2019;88:487‐514. - PubMed
-
- Mathieu M, Martin‐Jaular L, Lavieu G, Théry C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell‐to‐cell communication. Nat Cell Biol. 2019;21:9‐17. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
