Immune modulation attenuates infantile neuronal ceroid lipofuscinosis in mice before and after disease onset
- PMID: 33977263
- PMCID: PMC8098642
- DOI: 10.1093/braincomms/fcab047
Immune modulation attenuates infantile neuronal ceroid lipofuscinosis in mice before and after disease onset
Abstract
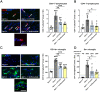
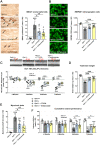
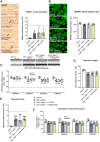
Targeting neuroinflammation in models for infantile and juvenile forms of neuronal ceroid lipofuscinosis (NCL, CLN disease) with the clinically established immunomodulators fingolimod and teriflunomide significantly attenuates the neurodegenerative phenotype when applied preventively, i.e. before the development of substantial neural damage and clinical symptoms. Here, we show that in a mouse model for the early onset and rapidly progressing CLN1 form, more complex clinical phenotypes like disturbed motor coordination and impaired visual acuity are also ameliorated by immunomodulation. Moreover, we show that the disease outcome can be attenuated even when fingolimod and teriflunomide treatment starts after disease onset, i.e. when neurodegeneration is ongoing and clinical symptoms are detectable. In detail, treatment with either drug led to a reduction in T-cell numbers and microgliosis in the CNS, although not to the same extent as upon preventive treatment. Pharmacological immunomodulation was accompanied by a reduction of axonal damage, neuron loss and astrogliosis in the retinotectal system and by reduced brain atrophy. Accordingly, the frequency of myoclonic jerks and disturbed motor coordination were attenuated. Overall, disease alleviation was remarkably substantial upon therapeutic treatment with both drugs, although less robust than upon preventive treatment. To test the relevance of putative immune-independent mechanisms of action in this model, we treated CLN1 mice lacking mature T- and B-lymphocytes. Immunodeficient CLN1 mice showed, as previously reported, an improved neurological phenotype in comparison with genuine CLN1 mice which could not be further alleviated by either of the drugs, reflecting a predominantly immune-related therapeutic mechanism of action. The present study supports and strengthens our previous view that repurposing clinically approved immunomodulators may alleviate the course of CLN1 disease in human patients, even though diagnosis usually occurs when symptoms have already emerged.
Keywords: T-lymphocytes; attenuation of disease; immunomodulation; infantile neuronal ceroid lipofuscinosis; neurodegeneration; neuroinflammation; preventive treatment.
© The Author(s) (2021). Published by Oxford University Press on behalf of the Guarantors of Brain.
Figures


References
-
- Mole SE, Anderson G, Band HA, et al.Clinical challenges and future therapeutic approaches for neuronal ceroid lipofuscinosis. Lancet Neurol. 2019;18(1):107–116. - PubMed
-
- Santavuori P. Neuronal ceroid-lipofuscinoses in childhood. Brain Dev. 1988;10(2):80–83. - PubMed
-
- Vesa J, Hellsten E, Verkruyse LA, et al.Mutations in the palmitoyl protein thioesterase gene causing infantile neuronal ceroid lipofuscinosis. Nature. 1995;376(6541):584–587. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
