Terminal Schwann cells at the human neuromuscular junction
- PMID: 33977269
- PMCID: PMC8093923
- DOI: 10.1093/braincomms/fcab081
Terminal Schwann cells at the human neuromuscular junction
Abstract
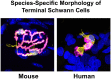
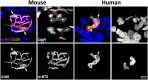
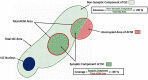
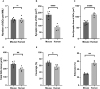
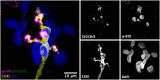
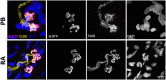
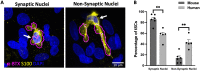
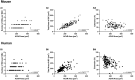
Terminal Schwann cells are non-myelinating glial cells localized to the neuromuscular junction. They play an important role in regulating many aspects of neuromuscular junction form and function, in health and during disease. However, almost all previous studies of mammalian terminal Schwann cells have used rodent models. Despite a growing awareness of differences in the cellular and molecular anatomy of rodent and human neuromuscular junctions, it remains unclear as to whether these differences also extend to the terminal Schwann cells. Here, we have adapted immunohistochemical protocols to facilitate visualization and comparative morphometric analyses of terminal Schwann cells at the human and mouse neuromuscular junction. We labelled terminal Schwann cells in the peroneus brevis muscle in six adult mice and five humans with antibodies against S100 protein. All human neuromuscular junctions were associated with at least one terminal Schwann cell, consistent with findings from other species, with an average of ∼1.7 terminal Schwann cells per neuromuscular junction in both humans and mice. In contrast, human terminal Schwann cells were significantly smaller than those of mice (P ≤ 0.01), in keeping with differences in overall synaptic size. Human terminal Schwann cell cytoplasm extended significantly beyond the synaptic boundaries of the neuromuscular junction, whereas terminal Schwann cells in mice were largely restricted to the synapse. Moreover, there was a significant difference in the location of terminal Schwann cell nuclei (P ≤ 0.01), with human terminal Schwann cells having their nuclear compartment located beyond the perimeter of the synapse more than the mouse. Taken together, these findings demonstrate that terminal Schwann cells at the human neuromuscular junction have notable differences in their morphology and synaptic relationships compared to mice. These fundamental differences need to be considered when translating the findings of both neuromuscular junction biology and pathology from rodents to humans.
Keywords: NMJ-morph; human; mouse; neuromuscular junction; terminal Schwann cell.
© The Author(s) (2021). Published by Oxford University Press on behalf of the Guarantors of Brain.
Figures

References
-
- Griffin JW, Thompson WJ.. Biology and pathology of nonmyelinating schwann cells. Glia. 2008;56(14):1518–1531. - PubMed
-
- Alvarez-Suarez P, Gawor M, Prószyński TJ.. Perisynaptic schwann cells - The multitasking cells at the developing neuromuscular junctions. Semin Cell Dev Biol. 2020;104:31–38. - PubMed
-
- Darabid H, St-Pierre-See A, Robitaille R.. Purinergic-dependent glial regulation of synaptic plasticity of competing terminals and synapse elimination at the neuromuscular junction. Cell Rep. 2018;25(8):2070–2082.e6. - PubMed
-
- Winlow W, Usherwood PNR.. Ultrastructural studies of normal and degenerating mouse neuromuscular junctions. J Neurocytol. 1975;4(4):377–394. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
