Severe Acute Respiratory Syndrome Coronavirus 2 Transmission in Intercollegiate Athletics Not Fully Mitigated With Daily Antigen Testing
- PMID: 33977295
- PMCID: PMC8136076
- DOI: 10.1093/cid/ciab343
Severe Acute Respiratory Syndrome Coronavirus 2 Transmission in Intercollegiate Athletics Not Fully Mitigated With Daily Antigen Testing
Abstract
Background: High-frequency, rapid-turnaround severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) testing continues to be proposed as a way of efficiently identifying and mitigating transmission in congregate settings. However, 2 SARS-CoV-2 outbreaks occurred among intercollegiate university athletic programs during the fall 2020 semester, despite mandatory directly observed daily antigen testing.
Methods: During the fall 2020 semester, athletes and staff in both programs were tested daily using Quidel's Sofia SARS Antigen Fluorescent Immunoassay, with positive antigen results requiring confirmatory testing with real-time reverse-transcription polymerase chain reaction. We used genomic sequencing to investigate transmission dynamics in these 2 outbreaks.
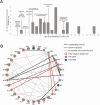
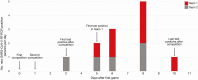
Results: In the first outbreak, 32 confirmed cases occurred within a university athletics program after the index patient attended a meeting while infectious, despite a negative antigen test on the day of the meeting. Among isolates sequenced from that outbreak, 24 (92%) of 26 were closely related, suggesting sustained transmission following an initial introduction event. In the second outbreak, 12 confirmed cases occurred among athletes from 2 university programs that faced each other in an athletic competition, despite receipt of negative antigen test results on the day of the competition. Sequences from both teams were closely related and distinct from viruses circulating in the community for team 1, suggesting transmission during intercollegiate competition in the community for team 2.
Conclusions: These findings suggest that antigen testing alone, even when mandated and directly observed, may not be sufficient as an intervention to prevent SARS-CoV-2 outbreaks in congregate settings, and they highlight the importance of vaccination to prevent SARS-CoV-2 outbreak in congregate settings.
Keywords: SARS-CoV-2; antigen testing; genomic epidemiology.
© The Author(s) 2021. Published by Oxford University Press for the Infectious Diseases Society of America. All rights reserved. For permissions, e-mail: journals.permissions@oup.com.
Figures



Update of
-
SARS-CoV-2 transmission in intercollegiate athletics not fully mitigated with daily antigen testing.medRxiv [Preprint]. 2021 Mar 6:2021.03.03.21252838. doi: 10.1101/2021.03.03.21252838. medRxiv. 2021. Update in: Clin Infect Dis. 2021 Jul 15;73(Suppl 1):S45-S53. doi: 10.1093/cid/ciab343. PMID: 33688665 Free PMC article. Updated. Preprint.
References
-
- Botti-Lodovico Y, Rosenberg E, Sabeti PC. Testing in a pandemic–improving access, coordination, and prioritization. N Engl J Med 2021; 384:197–9. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

