Delivery of systemic anti-cancer therapy during the COVID-19 pandemic
- PMID: 33977394
- PMCID: PMC8112878
- DOI: 10.1007/s11845-021-02631-1
Delivery of systemic anti-cancer therapy during the COVID-19 pandemic
Abstract
Background: The first confirmed case of COVID-19 in Ireland was on February 29th 2020. From March until late April, the number of cases increased exponentially. The delivery of anti-cancer therapy during the COVID-19 pandemic was extremely challenging. In order to balance the benefits of continuing anti-cancer therapy with the associated increased hospital visits, combined with the risk of COVID-19 infection, we undertook a series of system changes in the delivery of cancer care.
Methods: Patients who attended our dayward over a 4-month period were included. Data were obtained from patient and chemotherapy prescribing records. Patients were screened for symptoms of COVID-19 at two separate timepoints: prior to their visit via telephone, and using a symptom questionnaire on arrival at the hospital. If patients displayed COVID-19 symptoms, they were isolated and a viral swab arranged.
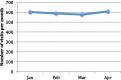
Results: A total of 456 patients attended from January 1st to April 30th. The numbers of visits from January to April were 601, 586, 575, and 607, respectively. During this period, there were 2369 patient visits to the dayward and 1953 (82%) intravenous regimens administered. Of the 416 visits that did not lead to treatment, 114 (27%) were scheduled non-treatment review visits, 194 (47%) treatments were held due to disease-related illness, and 108 (26%) treatments were held due to treatment-related complications. Screening measurements were implemented on March 18th due to rising COVID-19 prevalence in the general population. Overall, 53 treatments were held due to the screening process: 19 patients (36%) elicited COVID-19 symptoms via telephone screening; 34 patients (64%) were symptomatic in our pre-assessment area and referred for swabs, of which 4 were positive. Those with a negative swab were rescheduled for chemotherapy the following week.
Conclusions: With careful systematic changes, safe and continued delivery of systemic anti-cancer therapy during the COVID-19 pandemic is possible.
Keywords: COVID-19; Oncology; Screening; Treatment.
© 2021. The Author(s).
Figures
References
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous