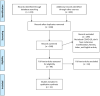
A systematic review of the histopathologic survey on skin biopsies in patients with Corona Virus Disease 2019 (COVID-19) who developed virus or drug-related mucocutaneous manifestations
- PMID: 33977531
- PMCID: PMC8239817
- DOI: 10.1111/exd.14384
A systematic review of the histopathologic survey on skin biopsies in patients with Corona Virus Disease 2019 (COVID-19) who developed virus or drug-related mucocutaneous manifestations
Abstract
The mucocutaneous manifestations of Corona Virus Disease 2019 (COVID-19) logically may reflect systemic visceral involvements. These findings are visible and easy to approach like biopsies for exact histopathologic evaluations. This systematic review was conducted to collect the mucocutaneous histopathologic data of COVID-19 patients for future investigations and interpretations. The COVID-19 dermatology resource of the Centre of Evidence-Based Dermatology (CEBD) at the University of Nottingham, PubMed, Scopus, Google Scholar and Medscape was searched for relevant English articles published by June 3, 2020. This review included 31 articles, involving 459 patients. The common primary virus-related mucocutaneous manifestations are easy to approach in the course of COVID-19. The authors of this study supposed dermatopathological findings as the predictors of the nature of potential systemic involvements and outcomes of COVID-19. Scrutinizing these findings can help with adopting more effective therapeutic and management strategies; nevertheless, this review found the severity and time of onset of symptoms not to be associated with the laboratory and histopathological findings. Deterioration of clinical conditions and laboratory tests was also not related to the histopathological findings. It is recommended that meta-analyses be conducted in the future to detail on these data for having more comprehensive and better conclusion.
Keywords: COVID-19; SARS-CoV-2; biopsy; coronavirus; cutaneous; dermatology; histopathology; mucocutaneous; pathology; skin; systematic review.
© 2021 John Wiley & Sons A/S. Published by John Wiley & Sons Ltd.
Conflict of interest statement
None declared.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous