Efficient and gentle delivery of molecules into cells with different elasticity via Progressive Mechanoporation
- PMID: 33977944
- PMCID: PMC8204113
- DOI: 10.1039/d0lc01224f
Efficient and gentle delivery of molecules into cells with different elasticity via Progressive Mechanoporation
Abstract
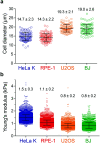
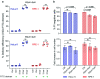
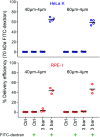
Intracellular delivery of cargo molecules such as membrane-impermeable proteins or drugs is crucial for cell treatment in biological and medical applications. Recently, microfluidic mechanoporation techniques have enabled transfection of previously inaccessible cells. These techniques create transient pores in the cell membrane by shear-induced or constriction contact-based rapid cell deformation. However, cells deform and recover differently from a given extent of shear stress or compression and it is unclear how the underlying mechanical properties affect the delivery efficiency of molecules into cells. In this study, we identify cell elasticity as a key mechanical determinant of delivery efficiency leading to the development of "progressive mechanoporation" (PM), a novel mechanoporation method that improves delivery efficiency into cells of different elasticity. PM is based on a multistage cell deformation, through a combination of hydrodynamic forces that pre-deform cells followed by their contact-based compression inside a PDMS-based device controlled by a pressure-based microfluidic controller. PM allows processing of small sample volumes (about 20 μL) with high-throughput (>10 000 cells per s), while controlling both operating pressure and flow rate for a reliable and reproducible cell treatment. We find that uptake of molecules of different sizes is correlated with cell elasticity whereby delivery efficiency of small and big molecules is favoured in more compliant and stiffer cells, respectively. A possible explanation for this opposite trend is a different size, number and lifetime of opened pores. Our data demonstrates that PM reliably and reproducibly delivers impermeable cargo of the size of small molecule inhibitors such as 4 kDa FITC-dextran with >90% efficiency into cells of different mechanical properties without affecting their viability and proliferation rates. Importantly, also much larger cargos such as a >190 kDa Cas9 protein-sgRNA complex are efficiently delivered high-lighting the biological, biomedical and clinical applicability of our findings.
Conflict of interest statement
The authors declare that they have no competing interests. TU Dresden and MPG has filed a patent application based on this work, in which S. G, R. G., A. U., J. M. and J. G. are listed as inventors.
Figures



References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

