A tractable covalent linker strategy for the production of immunogenic antigen-TLR7/8L bioconjugates
- PMID: 33977971
- PMCID: PMC9118693
- DOI: 10.1039/d1cc00795e
A tractable covalent linker strategy for the production of immunogenic antigen-TLR7/8L bioconjugates
Abstract
Despite the ease of production and improved safety profiles of recombinant vaccines, the inherently low immunogenicity of unadjuvanted proteins remains an impediment to their widespread adoption. The covalent tethering of TLR agonists to antigenic proteins offers a unique approach to co-deliver both constituents to the same cell-enhancing vaccine efficacy while minimizing reactogenicity. However, the paucity of simple and effective linker chemistries continues to hamper progress. Here, we present a modular, PEG-based linker system compatible with even extremely lipophilic and challenging TLR7/8 agonists. To advance the field and address previous obstacles, we offer the most straightforward and antigen-preserving linker system to date. These antigen-adjuvant conjugates enhance antigen-specific immune responses in mice, demonstrating the power of our approach within the context of modern vaccinology.
Conflict of interest statement
Conflicts of interest
There are no conflicts to declare.
Figures

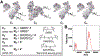
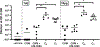
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

