Implementation of a Sample Pooling Strategy for the Direct Detection of SARS-CoV-2 by Real-Time Polymerase Chain Reaction During the COVID-19 Pandemic
- PMID: 33978164
- PMCID: PMC8136033
- DOI: 10.1093/ajcp/aqab035
Implementation of a Sample Pooling Strategy for the Direct Detection of SARS-CoV-2 by Real-Time Polymerase Chain Reaction During the COVID-19 Pandemic
Abstract
Objectives: To report our institutional experience in devising and implementing a pooling protocol and process for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) reverse transcription polymerase chain reaction (RT-PCR) testing over a 3-month period in the fall of 2020.
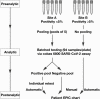
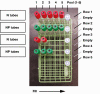
Methods: The widespread testing implemented in the United States for detecting SARS-CoV-2 infection in response to the coronavirus disease 2019 pandemic has led to a significant shortage of testing supplies and therefore has become a major impediment to the public health response. To date, several institutions have implemented sample pooling, but publications documenting these experiences are sparse. Nasal and nasopharyngeal samples collected from low-positivity (<5%) areas were tested in pools of five on the Roche cobas 6800 analyzer system. Routine SARS-CoV-2 RT-PCR turnaround times between sample collection to result reporting were monitored and compared before and after sample pooling implementation.
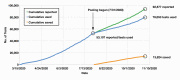
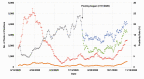
Results: A total of 4,131 sample pools were tested over a 3-month period (during which 39,770 RT-PCR results were reported from the Roche system), allowing our laboratory to save 13,824 tests, equivalent to a conservation rate of 35%. A 48-hour or less turnaround time was generally maintained throughout the pooling period.
Conclusions: Sample pooling offers a viable means to mitigate shortfalls of PCR testing supplies in the ongoing pandemic without significantly compromising overall turnaround times.
Keywords: COVID-19; PCR; Pooled testing; Pooling; RT-PCR; SARS-CoV-2; Test reagents.
© American Society for Clinical Pathology, 2021. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures
Similar articles
-
Comparing two sample pooling strategies for SARS-CoV-2 RNA detection for efficient screening of COVID-19.J Med Virol. 2021 May;93(5):2805-2809. doi: 10.1002/jmv.26632. Epub 2021 Mar 11. J Med Virol. 2021. PMID: 33107614
-
Pooled testing for COVID-19 diagnosis by real-time RT-PCR: A multi-site comparative evaluation of 5- & 10-sample pooling.Indian J Med Res. 2020 Jul & Aug;152(1 & 2):88-94. doi: 10.4103/ijmr.IJMR_2304_20. Indian J Med Res. 2020. PMID: 32893844 Free PMC article.
-
Evaluation of sample pooling for diagnosis of COVID-19 by real time-PCR: A resource-saving combat strategy.J Med Virol. 2021 Mar;93(3):1526-1531. doi: 10.1002/jmv.26475. Epub 2020 Sep 29. J Med Virol. 2021. PMID: 32869865
-
[SARS-CoV-2 and Microbiological Diagnostic Dynamics in COVID-19 Pandemic].Mikrobiyol Bul. 2020 Jul;54(3):497-509. doi: 10.5578/mb.69839. Mikrobiyol Bul. 2020. PMID: 32755524 Review. Turkish.
-
Alternative clinical specimens for the detection of SARS-CoV-2: A rapid review.Rev Med Virol. 2021 Jul;31(4):e2185. doi: 10.1002/rmv.2185. Epub 2020 Oct 22. Rev Med Virol. 2021. PMID: 33091200 Review.
Cited by
-
Validation of SARS-CoV-2 pooled testing for surveillance using the Panther Fusion® system: Impact of pool size, automation, and assay chemistry.PLoS One. 2022 Nov 7;17(11):e0276729. doi: 10.1371/journal.pone.0276729. eCollection 2022. PLoS One. 2022. PMID: 36342921 Free PMC article.
-
Real-world Evaluation of a Sample Pooling Strategy for Large-Scale Rapid COVID-19 Testing.J Clin Virol. 2022 Apr;149:105133. doi: 10.1016/j.jcv.2022.105133. Epub 2022 Mar 15. J Clin Virol. 2022. PMID: 35338949 Free PMC article.
-
Evaluation of the influenza and respiratory syncytial virus (RSV) targets in the AusDiagnostics SARS-CoV-2, Influenza and RSV 8-well assay: sample pooling increases testing throughput.Pathology. 2022 Jun;54(4):466-471. doi: 10.1016/j.pathol.2022.02.002. Epub 2022 Apr 21. Pathology. 2022. PMID: 35461715 Free PMC article.
-
SARS-CoV-2 surveillance by RT-qPCR-based pool testing of saliva swabs (lollipop method) at primary and special schools-A pilot study on feasibility and acceptability.PLoS One. 2022 Sep 13;17(9):e0274545. doi: 10.1371/journal.pone.0274545. eCollection 2022. PLoS One. 2022. PMID: 36099277 Free PMC article.
-
A method for campus-wide SARS-CoV-2 surveillance at a large public university.PLoS One. 2021 Dec 17;16(12):e0261230. doi: 10.1371/journal.pone.0261230. eCollection 2021. PLoS One. 2021. PMID: 34919584 Free PMC article.
References
-
- World Health Organization. Laboratory testing strategy recommendations for COVID-19: interim guidance, 21 March 2020. https://apps.who.int/iris/handle/10665/331509. Accessed December 7, 2020.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

