Identifying UK travellers at increased risk of developing pneumococcal infection: a novel algorithm
- PMID: 33978186
- PMCID: PMC8393689
- DOI: 10.1093/jtm/taab063
Identifying UK travellers at increased risk of developing pneumococcal infection: a novel algorithm
Abstract
Background: In 2016, the travel subcommittee of the UK Joint Committee on Vaccination and Immunisation (JCVI) recommended that 13-valent PCV (PCV13) could be offered to travellers aged over 65 years, visiting countries without infant PCV immunization programmes. This study aimed to identify, collate and review the available evidence to identify specific countries where UK travellers might be at an increased risk of developing pneumococcal infection. The data were then used to develop an algorithm, which could be used to facilitate implementation of the JCVI recommendation.
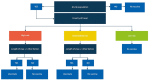
Methods: We conducted a systematic search of the published data available for pneumococcal disease, PCV vaccine implementation, coverage data and programme duration by country. The primary data sources used were World Health Organization databases and the International Vaccine Access Centre Vaccine Information and Epidemiology Window-hub database. Based on the algorithm, the countries were classified into 'high overall risk', 'intermediate overall risk' and 'low overall risk' from an adult traveller perspective. This could determine whether PCV13 should be recommended for UK adult travellers.
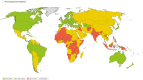
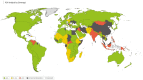
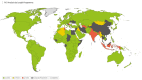
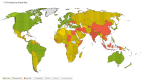
Results: A data search for a total of 228 countries was performed, with risk scores calculated for 188 countries. Overall, 45 countries were classified as 'high overall risk', 86 countries as 'intermediate overall risk', 57 countries as 'low overall risk' and 40 countries as 'unknown'.
Conclusion: To our knowledge this is the first attempt to categorize the risk to UK adult travellers of contracting pneumococcal infection in each country, globally. These findings could be used by national travel advisory bodies and providers of travel vaccines to identify travellers at increased risk of pneumococcal infection, who could be offered PCV immunization.
Keywords: Travel; pneumococcal disease; prevention; risk algorithm; traveller; vaccination.
© International Society of Travel Medicine 2021. Published by Oxford University Press.
Figures
Similar articles
-
Long-term Impact of a "3 + 0" Schedule for 7- and 13-Valent Pneumococcal Conjugate Vaccines on Invasive Pneumococcal Disease in Australia, 2002-2014.Clin Infect Dis. 2017 Jan 15;64(2):175-183. doi: 10.1093/cid/ciw720. Epub 2016 Oct 21. Clin Infect Dis. 2017. PMID: 27986682
-
Prevention of pneumococcal disease among infants and children - use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine - recommendations of the Advisory Committee on Immunization Practices (ACIP).MMWR Recomm Rep. 2010 Dec 10;59(RR-11):1-18. MMWR Recomm Rep. 2010. PMID: 21150868
-
Cost-effectiveness of conjugate pneumococcal vaccination in Singapore: comparing estimates for 7-valent, 10-valent, and 13-valent vaccines.Vaccine. 2011 Sep 2;29(38):6686-94. doi: 10.1016/j.vaccine.2011.06.091. Epub 2011 Jul 13. Vaccine. 2011. PMID: 21745516
-
Summary of evidence to reduce the two-dose infant priming schedule to a single dose of the 13-valent pneumococcal conjugate vaccine in the national immunisation programme in the UK.Lancet Infect Dis. 2021 Apr;21(4):e93-e102. doi: 10.1016/S1473-3099(20)30492-8. Epub 2020 Oct 29. Lancet Infect Dis. 2021. PMID: 33129426 Review.
-
Experience with pneumococcal polysaccharide conjugate vaccine (conjugated to CRM197 carrier protein) in children and adults.Clin Microbiol Infect. 2013 Oct;19 Suppl 1:1-9. doi: 10.1111/1469-0691.12320. Clin Microbiol Infect. 2013. PMID: 24083785 Review.
Cited by
-
The Ready-To-Go Questionnaire predicts health outcomes during travel: a smartphone application-based analysis.J Travel Med. 2023 Dec 28;30(8):taad117. doi: 10.1093/jtm/taad117. J Travel Med. 2023. PMID: 37669125 Free PMC article.
References
-
- Amin-Chowdhury Z, Iyanger N, Ramsay ME, Ladhani SN. Outbreaks of severe pneumococcal disease in closed settings in the conjugate vaccines era, 2010-2018: a systematic review to inform national guidance in the UK. J Infect 2019; 79:495–502. - PubMed
-
- Merck Sharp & Dohme Corp . (Whitehouse Station, NJ, USA) Pneumovax 23. Prescribing Information. 2020. Available at: https://www.merck.com/product/usa/pi_circulars/p/pneumovax_23/pneumovax_... (Accessed October 2020).
-
- GlaxoSmithKline . (Whitehouse Station, NJ, USA) Synflorix Summary of Product Characteristics. 2019. Available at: https://gskpro.com/content/dam/global/hcpportal/en_MT/PDF/Homepage/Produ... (Accessed October 2020).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

