Citicoline and Memory Function in Healthy Older Adults: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial
- PMID: 33978188
- PMCID: PMC8349115
- DOI: 10.1093/jn/nxab119
Citicoline and Memory Function in Healthy Older Adults: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial
Abstract
Background: Supplementation of citicoline (CDP-choline), a naturally occurring mononucleotide, has shown beneficial effects on memory function and behavior in populations with a wide range of impairments. However, few studies have investigated its effect in healthy older populations.
Objective: The objective of this study was to investigate the effects of citicoline (Cognizin®), on memory in healthy elderly populations with age-associated memory impairment (AAMI).
Methods: A total of 100 healthy men and women aged between 50 and 85 y with AAMI participated in this randomized, double-blind, placebo-controlled trial. Participants were randomized to receive placebo (n = 51) or citicoline (n = 49; 500 mg/d) for 12 wk. Memory function was assessed at baseline and end of the intervention (12 wk) using computerized tests (Cambridge Brain Sciences, Ontario, Canada). Safety measurements included adverse events query, body weight, blood pressure, and hematology and metabolic panel. Intent-to-treat analysis was conducted using ANCOVA for the primary and secondary outcome variables with Bonferroni correction for multiple comparisons.
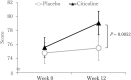
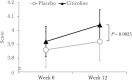
Results: A total of 99 out of 100 participants completed the study in its entirety. After the 12-wk intervention, participants supplemented with citicoline showed significantly greater improvements in secondary outcomes of episodic memory (assessed by the Paired Associate test), compared with those on placebo (mean: 0.15 vs. 0.06, respectively, P = 0.0025). Composite memory (secondary outcome), calculated using the scores of 4 memory tests, also significantly improved to a greater extent following citicoline supplementation (mean: 3.78) compared with placebo (mean: 0.72, P = 0.0052).
Conclusions: Dietary supplementation of citicoline for 12 wk improved overall memory performance, especially episodic memory, in healthy older males and females with AAMI. The findings suggest that regular consumption of citicoline may be safe and potentially beneficial against memory loss due to aging. This trial was registered at clinicaltrials.gov as NCT03369925.
Keywords: 5′-cytidine diphosphate choline; aging; brain; citicoline; memory loss.
© The Author(s) 2021. Published by Oxford University Press on behalf of the American Society for Nutrition.
Figures
Similar articles
-
Double-blind placebo-controlled study with citicoline in APOE genotyped Alzheimer's disease patients. Effects on cognitive performance, brain bioelectrical activity and cerebral perfusion.Methods Find Exp Clin Pharmacol. 1999 Nov;21(9):633-44. Methods Find Exp Clin Pharmacol. 1999. PMID: 10669911 Clinical Trial.
-
Citicoline improves verbal memory in aging.Arch Neurol. 1996 May;53(5):441-8. doi: 10.1001/archneur.1996.00550050071026. Arch Neurol. 1996. PMID: 8624220 Clinical Trial.
-
A Randomized, Double-Blind, Placebo-Controlled Trial of Citicoline in Patients with Alcohol Use Disorder.Alcohol Clin Exp Res. 2019 Feb;43(2):317-323. doi: 10.1111/acer.13928. Epub 2018 Dec 24. Alcohol Clin Exp Res. 2019. PMID: 30457668 Free PMC article. Clinical Trial.
-
"Brain-specific" nutrients: a memory cure?Nutrition. 2003 Nov-Dec;19(11-12):957-75. doi: 10.1016/s0899-9007(03)00024-8. Nutrition. 2003. PMID: 14624946 Review.
-
Citicoline for Supporting Memory in Aging Humans.Aging Dis. 2023 Aug 1;14(4):1184-1195. doi: 10.14336/AD.2022.0913. Aging Dis. 2023. PMID: 37196134 Free PMC article. Review.
Cited by
-
The Impact of Supplements on Recovery After Peripheral Nerve Injury: A Review of the Literature.Cureus. 2022 May 19;14(5):e25135. doi: 10.7759/cureus.25135. eCollection 2022 May. Cureus. 2022. PMID: 35733475 Free PMC article. Review.
-
Higher intake of certain nutrients among older adults is associated with better cognitive function: an analysis of NHANES 2011-2014.BMC Nutr. 2023 Dec 5;9(1):142. doi: 10.1186/s40795-023-00802-0. BMC Nutr. 2023. PMID: 38053133 Free PMC article.
-
Long-Term Food Variety and Dietary Patterns Are Associated with Frailty among Chinese Older Adults: A Cohort Study Based on CLHLS from 2014 to 2018.Nutrients. 2022 Oct 13;14(20):4279. doi: 10.3390/nu14204279. Nutrients. 2022. PMID: 36296962 Free PMC article.
-
Investigating nutrient biomarkers of healthy brain aging: a multimodal brain imaging study.NPJ Aging. 2024 May 21;10(1):27. doi: 10.1038/s41514-024-00150-8. NPJ Aging. 2024. PMID: 38773079 Free PMC article.
-
Efficacy and Safety of Panax ginseng Sprout Extract in Subjective Memory Impairment: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial.Nutrients. 2024 Jun 19;16(12):1952. doi: 10.3390/nu16121952. Nutrients. 2024. PMID: 38931306 Free PMC article. Clinical Trial.
References
-
- United Nations, Department of Economic and Social Affairs, Population Division.. World Population Prospects 2019: highlights. New York: United Nations; 2019.
-
- Prince M, Albanese E, Guerchet M, Prina M. World Alzheimer Report 2014: dementia and risk reduction. London: Alzheimer's Disease International (ADI); 2014.
-
- Vauzour D, Camprubi-Robles M, Miquel-Kergoat S, Andres-Lacueva C, Bánáti D, Barberger-Gateau P, Bowman GL, Caberlotto L, Clarke R, Hogervorst Eet al. . Nutrition for the ageing brain: towards evidence for an optimal diet. Ageing Res Rev. 2017;35:222–40. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

