Dysbalance of ACE2 levels - a possible cause for severe COVID-19 outcome in COPD
- PMID: 33978304
- PMCID: PMC8239572
- DOI: 10.1002/cjp2.224
Dysbalance of ACE2 levels - a possible cause for severe COVID-19 outcome in COPD
Abstract
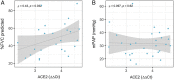
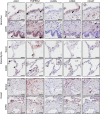
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) poses a serious threat to healthcare systems worldwide. Binding of the virus to angiotensin-converting enzyme 2 (ACE2) is an important step in the infection mechanism. However, it is unknown if ACE2 expression in patients with chronic lung diseases (CLDs), such as chronic obstructive pulmonary disease (COPD), idiopathic pulmonary arterial hypertension (IPAH), or pulmonary fibrosis (PF), is changed as compared to controls. We used lung samples from patients with COPD (n = 28), IPAH (n = 10), and PF (n = 10) as well as healthy control donor (n = 10) tissue samples to investigate the expression of ACE2 and related cofactors that might influence the course of SARS-CoV-2 infection. Expression levels of the ACE2 receptor, the putative receptor CD147/BSG, and the viral entry cofactors TMPRSS2 (transmembrane serine protease 2), EZR, and FURIN were determined by quantitative PCR and in open-access RNA sequencing datasets. Immunohistochemical and single-cell RNA sequencing (scRNAseq) analyses were used for localization and coexpression, respectively. Soluble ACE2 (sACE2) plasma levels were analyzed by enzyme-linked immunosorbent assay. In COPD as compared to donor, IPAH, and PF lung tissue, gene expression of ACE2, TMPRSS2, and EZR was significantly elevated, but circulating sACE2 levels were significantly reduced in COPD and PF plasma compared to healthy control and IPAH plasma samples. Lung tissue expressions of FURIN and CD147/BSG were downregulated in COPD. None of these changes were associated with changes in pulmonary hemodynamics. Histological analysis revealed coexpression of ACE2, TMPRSS2, and Ezrin in bronchial regions and epithelial cells. This was confirmed by scRNAseq analysis. There were no significant expression changes of the analyzed molecules in the lung tissue of IPAH and idiopathic PF as compared to control. In conclusion, we reveal increased ACE2 and TMPRSS2 expression in lung tissue with a concomitant decrease of protective sACE2 in COPD patients. These changes represent the possible risk factors for an increased susceptibility of COPD patients to SARS-CoV-2 infection.
Keywords: ACE2; COPD; COVID-19; SARS-CoV-2; TMPRSS2; chronic lung disease; chronic obstructive pulmonary disease; pulmonary fibrosis; pulmonary hypertension.
© 2021 The Authors. The Journal of Pathology: Clinical Research published by The Pathological Society of Great Britain and Ireland & John Wiley & Sons, Ltd.
Figures
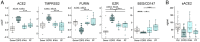


Similar articles
-
Pulmonary, cardiac and renal distribution of ACE2, furin, TMPRSS2 and ADAM17 in rats with heart failure: Potential implication for COVID-19 disease.J Cell Mol Med. 2021 Apr;25(8):3840-3855. doi: 10.1111/jcmm.16310. Epub 2021 Mar 4. J Cell Mol Med. 2021. PMID: 33660945 Free PMC article.
-
Structural variations and expression profiles of the SARS-CoV-2 host invasion genes in lung cancer.J Med Virol. 2020 Nov;92(11):2637-2647. doi: 10.1002/jmv.26107. Epub 2020 Jun 19. J Med Virol. 2020. PMID: 32492203 Free PMC article.
-
Dysregulation of COVID-19 related gene expression in the COPD lung.Respir Res. 2021 May 29;22(1):164. doi: 10.1186/s12931-021-01755-3. Respir Res. 2021. PMID: 34051791 Free PMC article.
-
Contributions of human ACE2 and TMPRSS2 in determining host-pathogen interaction of COVID-19.J Genet. 2021;100(1):12. doi: 10.1007/s12041-021-01262-w. J Genet. 2021. PMID: 33707363 Free PMC article. Review.
-
Bridging Cancer and COVID-19: The Complex Interplay of ACE2 and TMPRSS2.Cancer Med. 2025 Apr;14(7):e70829. doi: 10.1002/cam4.70829. Cancer Med. 2025. PMID: 40145441 Free PMC article. Review.
Cited by
-
The Renin-Angiotensin System as a Component of Biotrauma in Acute Respiratory Distress Syndrome.Front Physiol. 2022 Apr 13;12:806062. doi: 10.3389/fphys.2021.806062. eCollection 2021. Front Physiol. 2022. PMID: 35498160 Free PMC article. Review.
-
COVID-19 in Patients with Chronic Lung Disease.Clin Chest Med. 2023 Jun;44(2):385-393. doi: 10.1016/j.ccm.2022.11.013. Epub 2022 Nov 22. Clin Chest Med. 2023. PMID: 37085227 Free PMC article. Review.
-
Sex difference in circulating soluble form of ACE2 protein in moderate and severe COVID-19 and healthy controls.Front Med (Lausanne). 2022 Dec 8;9:1058120. doi: 10.3389/fmed.2022.1058120. eCollection 2022. Front Med (Lausanne). 2022. PMID: 36569121 Free PMC article.
-
Mechanisms of pulmonary vascular dysfunction in pulmonary hypertension and implications for novel therapies.Am J Physiol Heart Circ Physiol. 2022 May 1;322(5):H702-H724. doi: 10.1152/ajpheart.00021.2022. Epub 2022 Feb 25. Am J Physiol Heart Circ Physiol. 2022. PMID: 35213243 Free PMC article. Review.
-
Chronic Obstructive Pulmonary Disease is Not Associated with In-Hospital Mortality in COVID-19: An Observational Cohort Analysis.Int J Chron Obstruct Pulmon Dis. 2022 Dec 19;17:3111-3121. doi: 10.2147/COPD.S386463. eCollection 2022. Int J Chron Obstruct Pulmon Dis. 2022. PMID: 36570857 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

