Selective cutoff reporting in studies of the accuracy of the Patient Health Questionnaire-9 and Edinburgh Postnatal Depression Scale: Comparison of results based on published cutoffs versus all cutoffs using individual participant data meta-analysis
- PMID: 33978306
- PMCID: PMC8412225
- DOI: 10.1002/mpr.1873
Selective cutoff reporting in studies of the accuracy of the Patient Health Questionnaire-9 and Edinburgh Postnatal Depression Scale: Comparison of results based on published cutoffs versus all cutoffs using individual participant data meta-analysis
Abstract
Objectives: Selectively reported results from only well-performing cutoffs in diagnostic accuracy studies may bias estimates in meta-analyses. We investigated cutoff reporting patterns for the Patient Health Questionnaire-9 (PHQ-9; standard cutoff 10) and Edinburgh Postnatal Depression Scale (EPDS; no standard cutoff, commonly used 10-13) and compared accuracy estimates based on published cutoffs versus all cutoffs.
Methods: We conducted bivariate random effects meta-analyses using individual participant data to compare accuracy from published versus all cutoffs.
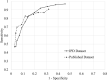
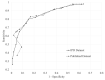
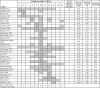
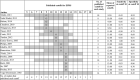
Results: For the PHQ-9 (30 studies, N = 11,773), published results underestimated sensitivity for cutoffs below 10 (median difference: -0.06) and overestimated for cutoffs above 10 (median difference: 0.07). EPDS (19 studies, N = 3637) sensitivity estimates from published results were similar for cutoffs below 10 (median difference: 0.00) but higher for cutoffs above 13 (median difference: 0.14). Specificity estimates from published and all cutoffs were similar for both tools. The mean cutoff of all reported cutoffs in PHQ-9 studies with optimal cutoff below 10 was 8.8 compared to 11.8 for those with optimal cutoffs above 10. Mean for EPDS studies with optimal cutoffs below 10 was 9.9 compared to 11.8 for those with optimal cutoffs greater than 10.
Conclusion: Selective cutoff reporting was more pronounced for the PHQ-9 than EPDS.
Keywords: diagnostic test accuracy; individual participant data meta-analysis; meta-analysis; publication bias; selective cutoff reporting.
© 2021 The Authors. International Journal of Methods in Psychiatric Research published by John Wiley & Sons Ltd.
Conflict of interest statement
All authors have completed the ICJME uniform disclosure form and declare: no support from any organization for the submitted work; no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years with the following exceptions: Dr. Tonelli declares that he has received a grant from Merck Canada, outside the submitted work. Dr. Vigod declares that she receives royalties from UpToDate, outside the submitted work. Dr. Beck declares that she receives royalties for her Postpartum Depression Screening Scale published by Western Psychological Services. Dr. Inagaki declares that he has received a grant from Novartis Pharma, and personal fees from Meiji, Mochida, Takeda, Novartis, Yoshitomi, Pfizer, Eisai, Otsuka, MSD, Technomics, and Sumitomo Dainippon, all outside of the submitted work. Dr. Ismail declares that she has received honorarium for speaker fees for educational lectures for Sanofi, Sunovion, Janssen and Novo Nordisk. All authors declare no other relationships or activities that could appear to have influenced the submitted work. No funder had any role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Figures
Similar articles
-
Selective Cutoff Reporting in Studies of Diagnostic Test Accuracy: A Comparison of Conventional and Individual-Patient-Data Meta-Analyses of the Patient Health Questionnaire-9 Depression Screening Tool.Am J Epidemiol. 2017 May 15;185(10):954-964. doi: 10.1093/aje/kww191. Am J Epidemiol. 2017. PMID: 28419203 Free PMC article.
-
An empirical comparison of three methods for multiple cutoff diagnostic test meta-analysis of the Patient Health Questionnaire-9 (PHQ-9) depression screening tool using published data vs individual level data.Res Synth Methods. 2020 Nov;11(6):833-848. doi: 10.1002/jrsm.1443. Epub 2020 Sep 13. Res Synth Methods. 2020. PMID: 32896096
-
'Optimal' cutoff selection in studies of depression screening tool accuracy using the PHQ-9, EPDS, or HADS-D: A meta-research study.Int J Methods Psychiatr Res. 2023 Sep;32(3):e1956. doi: 10.1002/mpr.1956. Epub 2022 Dec 3. Int J Methods Psychiatr Res. 2023. PMID: 36461893 Free PMC article. Review.
-
The diagnostic accuracy of the Patient Health Questionnaire-2 (PHQ-2), Patient Health Questionnaire-8 (PHQ-8), and Patient Health Questionnaire-9 (PHQ-9) for detecting major depression: protocol for a systematic review and individual patient data meta-analyses.Syst Rev. 2014 Oct 27;3:124. doi: 10.1186/2046-4053-3-124. Syst Rev. 2014. PMID: 25348422 Free PMC article.
-
Depression prevalence based on the Edinburgh Postnatal Depression Scale compared to Structured Clinical Interview for DSM DIsorders classification: Systematic review and individual participant data meta-analysis.Int J Methods Psychiatr Res. 2021 Mar;30(1):e1860. doi: 10.1002/mpr.1860. Epub 2020 Oct 22. Int J Methods Psychiatr Res. 2021. PMID: 33089942 Free PMC article.
Cited by
-
The overlapping relationship among depression, anxiety, and somatic symptom disorder and its impact on the quality of life of people with epilepsy.Ther Adv Neurol Disord. 2022 Dec 8;15:17562864221138147. doi: 10.1177/17562864221138147. eCollection 2022. Ther Adv Neurol Disord. 2022. PMID: 36518552 Free PMC article.
-
Individual participant data meta-analysis to compare EPDS accuracy to detect major depression with and without the self-harm item.Sci Rep. 2023 Mar 10;13(1):4026. doi: 10.1038/s41598-023-29114-w. Sci Rep. 2023. PMID: 36899016 Free PMC article.
-
Sensitivity and specificity of the Patient Health Questionnaire (PHQ-9, PHQ-8, PHQ-2) and General Anxiety Disorder scale (GAD-7, GAD-2) for depression and anxiety diagnosis: a cross-sectional study in a Peruvian hospital population.BMJ Open. 2023 Sep 15;13(9):e076193. doi: 10.1136/bmjopen-2023-076193. BMJ Open. 2023. PMID: 37714674 Free PMC article.
-
An empirical comparison of statistical methods for multiple cut-off diagnostic test accuracy meta-analysis of the Edinburgh postnatal depression scale (EPDS) depression screening tool using published results vs individual participant data.BMC Med Res Methodol. 2024 Feb 1;24(1):28. doi: 10.1186/s12874-023-02134-w. BMC Med Res Methodol. 2024. PMID: 38302928 Free PMC article.
-
Polysocial risk factors and trajectories of antenatal moderate-to-severe depressive symptoms: a retrospective cohort study in Shenzhen, China.BMC Med. 2025 Jul 31;23(1):451. doi: 10.1186/s12916-025-04290-w. BMC Med. 2025. PMID: 40745544 Free PMC article.
References
-
- Amoozegar, F., Patten, S. B., Becker, W. J., Bulloch, A. G. M., Fiest, K. M., Davenport, W. J., Carroll, C. R., & Jette, N. (2017). The prevalence of depression and the accuracy of depression screening tools in migraine patients. General Hospital Psychiatry, 48, 25–31. 10.1016/j.genhosppsych.2017.06.006 - DOI - PubMed
-
- Bakare, M. O., Okoye, J. O., & Obindo, J. T. (2014). Introducing depression and developmental screenings into the national programme on immunization (NPI) in southeast Nigeria: An experimental cross‐sectional assessment. General Hospital Psychiatry, 36(1), 105–112. 10.1016/j.genhosppsych.2013.09.005 - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

