Os(II)-Bridged Polyarginine Conjugates: The Additive Effects of Peptides in Promoting or Preventing Permeation in Cells and Multicellular Tumor Spheroids
- PMID: 33978399
- PMCID: PMC8277133
- DOI: 10.1021/acs.inorgchem.1c00769
Os(II)-Bridged Polyarginine Conjugates: The Additive Effects of Peptides in Promoting or Preventing Permeation in Cells and Multicellular Tumor Spheroids
Abstract
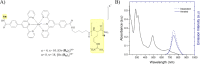
The preparation of two polyarginine conjugates of the complex Os(II) [bis-(4'-(4-carboxyphenyl)-2,2':6',2″-terpyridine)] [Os-(Rn)2]x+ (n = 4 and 8; x = 10 and 18) is reported, to explore whether the R8 peptide sequence that promotes cell uptake requires a contiguous amino acid sequence for membrane permeation or if this can be accomplished in a linearly bridged structure with the additive effect of shorter peptide sequences. The conjugates exhibit NIR emission centered at 754 nm and essentially oxygen-insensitive emission with a lifetime of 89 ns in phosphate-buffered saline. The uptake, distribution, and cytotoxicity of the parent complex and peptide derivatives were compared in 2D cell monolayers and a three-dimensional (3D) multicellular tumor spheroid (MCTS) model. Whereas, the bis-octaarginine sequences were impermeable to cells and spheroids, and the bis-tetraarginine conjugate showed excellent cellular uptake and accumulation in two 2D monolayer cell lines and remarkable in-depth penetration of 3D MCTSs of pancreatic cancer cells. Overall, the data indicates that cell permeability can be promoted via non-contiguous sequences of arginine residues bridged across the metal centre.
Conflict of interest statement
The authors declare no competing financial interest.
Figures







References
-
- Dolan C.; Burke C. S.; Byrne A.; Keyes T. E.. Cellular Uptake and Sensing Capability of Transition Metal Peptide Conjugates. In Inorganic and Organometallic Transition Metal Complexes with Biological Molecules and Living Cell; Elsevier, 2017, pp 55–89.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

