Inferring thalamocortical monosynaptic connectivity in vivo
- PMID: 33978507
- PMCID: PMC8285656
- DOI: 10.1152/jn.00591.2020
Inferring thalamocortical monosynaptic connectivity in vivo
Abstract
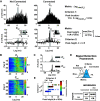
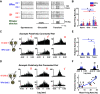
As the tools to simultaneously record electrophysiological signals from large numbers of neurons within and across brain regions become increasingly available, this opens up for the first time the possibility of establishing the details of causal relationships between monosynaptically connected neurons and the patterns of neural activation that underlie perception and behavior. Although recorded activity across synaptically connected neurons has served as the cornerstone for much of what we know about synaptic transmission and plasticity, this has largely been relegated to ex vivo preparations that enable precise targeting under relatively well-controlled conditions. Analogous studies in vivo, where image-guided targeting is often not yet possible, rely on indirect, data-driven measures, and as a result such studies have been sparse and the dependence upon important experimental parameters has not been well studied. Here, using in vivo extracellular single-unit recordings in the topographically aligned rodent thalamocortical pathway, we sought to establish a general experimental and computational framework for inferring synaptic connectivity. Specifically, attacking this problem within a statistical signal detection framework utilizing experimentally recorded data in the ventral-posterior medial (VPm) region of the thalamus and the homologous region in layer 4 of primary somatosensory cortex (S1) revealed a trade-off between network activity levels needed for the data-driven inference and synchronization of nearby neurons within the population that results in masking of synaptic relationships. Here, we provide a framework for establishing connectivity in multisite, multielectrode recordings based on statistical inference, setting the stage for large-scale assessment of synaptic connectivity within and across brain structures.NEW & NOTEWORTHY Despite the fact that all brain function relies on the long-range transfer of information across different regions, the tools enabling us to measure connectivity across brain structures are lacking. Here, we provide a statistical framework for identifying and assessing potential monosynaptic connectivity across neuronal circuits from population spiking activity that generalizes to large-scale recording technologies that will help us to better understand the signaling within networks that underlies perception and behavior.
Keywords: causality; cross correlation; inference; signal detection; thalamocortical circuit.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures







Similar articles
-
Multiple Distinct Timescales of Rapid Sensory Adaption in the Thalamocortical Circuit.J Neurosci. 2025 Apr 2;45(14):e1057242025. doi: 10.1523/JNEUROSCI.1057-24.2025. J Neurosci. 2025. PMID: 39933928
-
Thalamic state influences timing precision in the thalamocortical circuit.J Neurophysiol. 2021 May 1;125(5):1833-1850. doi: 10.1152/jn.00261.2020. Epub 2021 Mar 24. J Neurophysiol. 2021. PMID: 33760642 Free PMC article.
-
Ventral posterolateral and ventral posteromedial thalamocortical neurons have distinct physiological properties.J Neurophysiol. 2023 Dec 1;130(6):1492-1507. doi: 10.1152/jn.00525.2022. Epub 2023 Nov 8. J Neurophysiol. 2023. PMID: 37937368 Free PMC article.
-
Spike timing and synaptic dynamics at the awake thalamocortical synapse.Prog Brain Res. 2005;149:91-105. doi: 10.1016/S0079-6123(05)49008-1. Prog Brain Res. 2005. PMID: 16226579 Review.
-
Plasticity and stability of somatosensory maps in thalamus and cortex.Curr Opin Neurobiol. 2000 Aug;10(4):494-7. doi: 10.1016/s0959-4388(00)00112-4. Curr Opin Neurobiol. 2000. PMID: 10981619 Review.
Cited by
-
In Vivo Characterization of Intracortical Probes with Focused Ion Beam-Etched Nanopatterned Topographies.Micromachines (Basel). 2024 Feb 17;15(2):286. doi: 10.3390/mi15020286. Micromachines (Basel). 2024. PMID: 38399014 Free PMC article.
-
A machine learning approach for real-time cortical state estimation.J Neural Eng. 2024 Feb 1;21(1):10.1088/1741-2552/ad1f7b. doi: 10.1088/1741-2552/ad1f7b. J Neural Eng. 2024. PMID: 38232377 Free PMC article.
-
Functional interactions among neurons within single columns of macaque V1.Elife. 2022 Nov 2;11:e79322. doi: 10.7554/eLife.79322. Elife. 2022. PMID: 36321687 Free PMC article.
-
Multiple Distinct Timescales of Rapid Sensory Adaption in the Thalamocortical Circuit.J Neurosci. 2025 Apr 2;45(14):e1057242025. doi: 10.1523/JNEUROSCI.1057-24.2025. J Neurosci. 2025. PMID: 39933928
-
Visual Corticotectal Neurons in Awake Rabbits: Receptive Fields and Driving Monosynaptic Thalamocortical Inputs.J Neurosci. 2024 May 8;44(19):e1945232024. doi: 10.1523/JNEUROSCI.1945-23.2024. J Neurosci. 2024. PMID: 38485258 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

