Maternal exposures and the infant gut microbiome: a systematic review with meta-analysis
- PMID: 33978558
- PMCID: PMC8276657
- DOI: 10.1080/19490976.2021.1897210
Maternal exposures and the infant gut microbiome: a systematic review with meta-analysis
Abstract
Early life, including the establishment of the intestinal microbiome, represents a critical window of growth and development. Postnatal factors affecting the microbiome, including mode of delivery, feeding type, and antibiotic exposure have been widely investigated, but questions remain regarding the influence of exposures in utero on infant gut microbiome assembly. This systematic review aimed to synthesize evidence on exposures before birth, which affect the early intestinal microbiome. Five databases were searched in August 2019 for studies exploring pre-pregnancy or pregnancy 'exposure' data in relation to the infant microbiome. Of 1,441 publications identified, 76 were included. Factors reported influencing microbiome composition and diversity included maternal antibiotic and probiotic uses, dietary intake, pre-pregnancy body mass index (BMI), gestational weight gain (GWG), diabetes, mood, and others. Eleven studies contributed to three meta-analyses quantifying associations between maternal intrapartum antibiotic exposure (IAP), BMI and GWG, and infant microbiome alpha diversity (Shannon Index). IAP, maternal overweight/obesity and excessive GWG were all associated with reduced diversity. Most studies were observational, few included early recruitment or longitudinal follow-up, and the timing, frequency, and methodologies related to stool sampling and analysis were variable. Standardization and collaboration are imperative to enhance understanding in this complex and rapidly evolving area.
Keywords: Pregnancy; child health; gut microbiome diversity; infant gut microbiome; systematic review; women’s health.
Figures
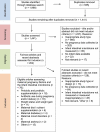

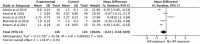
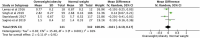
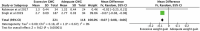

References
-
- Milani C, Duranti S, Bottacini F, Casey E, Turroni F, Mahony J, Belzer C, Delgado Palacio S, Arboleya Montes S, Mancabelli L, et al. The first microbial colonizers of the human gut: composition, activities, and health implications of the infant gut microbiota. Microbiol Mol Biol Rev. 2017;81(4):12. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
