Structural basis for allosteric control of the SERCA-Phospholamban membrane complex by Ca2+ and phosphorylation
- PMID: 33978571
- PMCID: PMC8184213
- DOI: 10.7554/eLife.66226
Structural basis for allosteric control of the SERCA-Phospholamban membrane complex by Ca2+ and phosphorylation
Abstract
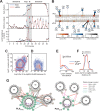
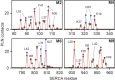
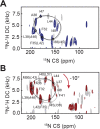
Phospholamban (PLN) is a mini-membrane protein that directly controls the cardiac Ca2+-transport response to β-adrenergic stimulation, thus modulating cardiac output during the fight-or-flight response. In the sarcoplasmic reticulum membrane, PLN binds to the sarco(endo)plasmic reticulum Ca2+-ATPase (SERCA), keeping this enzyme's function within a narrow physiological window. PLN phosphorylation by cAMP-dependent protein kinase A or increase in Ca2+ concentration reverses the inhibitory effects through an unknown mechanism. Using oriented-sample solid-state NMR spectroscopy and replica-averaged NMR-restrained structural refinement, we reveal that phosphorylation of PLN's cytoplasmic regulatory domain signals the disruption of several inhibitory contacts at the transmembrane binding interface of the SERCA-PLN complex that are propagated to the enzyme's active site, augmenting Ca2+ transport. Our findings address long-standing questions about SERCA regulation, epitomizing a signal transduction mechanism operated by posttranslationally modified bitopic membrane proteins.
Keywords: E. coli; membrane proteins; molecular biophysics; oriented solid; state NMR; structural biology; topological allostery.
© 2021, Weber et al.
Conflict of interest statement
DW, UR, SW, EL, TG, MG, RC, DT, AD, GV No competing interests declared
Figures



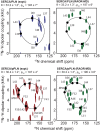
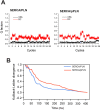
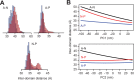
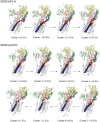
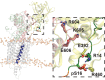

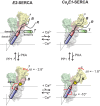

Similar articles
-
Allosteric regulation of SERCA by phosphorylation-mediated conformational shift of phospholamban.Proc Natl Acad Sci U S A. 2013 Oct 22;110(43):17338-43. doi: 10.1073/pnas.1303006110. Epub 2013 Oct 7. Proc Natl Acad Sci U S A. 2013. PMID: 24101520 Free PMC article.
-
Pathological mutations in the phospholamban cytoplasmic region affect its topology and dynamics modulating the extent of SERCA inhibition.Biochim Biophys Acta Biomembr. 2024 Oct;1866(7):184370. doi: 10.1016/j.bbamem.2024.184370. Epub 2024 Jul 8. Biochim Biophys Acta Biomembr. 2024. PMID: 38986894
-
Rheostatic Regulation of the SERCA/Phospholamban Membrane Protein Complex Using Non-Coding RNA and Single-Stranded DNA oligonucleotides.Sci Rep. 2015 Aug 21;5:13000. doi: 10.1038/srep13000. Sci Rep. 2015. PMID: 26292938 Free PMC article.
-
Phospholamban and sarcolipin: Are they functionally redundant or distinct regulators of the Sarco(Endo)Plasmic Reticulum Calcium ATPase?J Mol Cell Cardiol. 2016 Feb;91:81-91. doi: 10.1016/j.yjmcc.2015.12.030. Epub 2015 Dec 29. J Mol Cell Cardiol. 2016. PMID: 26743715 Free PMC article. Review.
-
Interaction sites among phospholamban, sarcolipin, and the sarco(endo)plasmic reticulum Ca(2+)-ATPase.Biochem Biophys Res Commun. 2008 Apr 25;369(1):188-94. doi: 10.1016/j.bbrc.2007.11.098. Epub 2007 Nov 29. Biochem Biophys Res Commun. 2008. PMID: 18053795 Review.
Cited by
-
Probing the formation of a hetero-dimeric membrane transport complex with dual in vitro and in silico mutagenesis.Chem Sci. 2024 Aug 6;15(35):14310-22. doi: 10.1039/d4sc02915a. Online ahead of print. Chem Sci. 2024. PMID: 39156929 Free PMC article.
-
Identification of core allosteric sites through temperature- and nucleus-invariant chemical shift covariance.Biophys J. 2022 Jun 7;121(11):2035-2045. doi: 10.1016/j.bpj.2022.05.004. Epub 2022 May 10. Biophys J. 2022. PMID: 35538664 Free PMC article.
-
β-adrenergic regulation of Ca2+ signaling in heart cells.Biophys Rep. 2024 Oct 31;10(5):274-282. doi: 10.52601/bpr.2024.240906. Biophys Rep. 2024. PMID: 39539286 Free PMC article.
-
Blocking phospholamban with VHH intrabodies enhances contractility and relaxation in heart failure.Nat Commun. 2022 May 31;13(1):3018. doi: 10.1038/s41467-022-29703-9. Nat Commun. 2022. PMID: 35641497 Free PMC article.
-
Allosteric Modulation of SERCA Pumps in Health and Disease: Structural Dynamics, Posttranslational Modifications, and Therapeutic Potential.J Mol Biol. 2025 May 9:169200. doi: 10.1016/j.jmb.2025.169200. Online ahead of print. J Mol Biol. 2025. PMID: 40349954 Review.
References
-
- Abraham MJ, Murtola T, Schulz R, Páll S, Smith JC, Hess B, Lindahl E. GROMACS: high performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX. 2015;1-2:19–25. doi: 10.1016/j.softx.2015.06.001. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

