Delayed Localized Hypersensitivity Reactions to the Moderna COVID-19 Vaccine: A Case Series
- PMID: 33978670
- PMCID: PMC8117061
- DOI: 10.1001/jamadermatol.2021.1214
Delayed Localized Hypersensitivity Reactions to the Moderna COVID-19 Vaccine: A Case Series
Abstract
Importance: In response to the coronavirus disease 2019 (COVID-19) pandemic, 2 mRNA vaccines (Pfizer-BioNTech and Moderna) received emergency use authorization from the US Food and Drug Administration in December 2020. Some patients in the US have developed delayed localized cutaneous vaccine reactions that have been dubbed "COVID arm."
Objective: To describe the course of localized cutaneous injection-site reactions to the Moderna COVID-19 vaccine, subsequent reactions to the second vaccine dose, and to characterize the findings of histopathologic examination of the reaction.
Design, setting, and participants: This retrospective case series study was performed at Yale New Haven Hospital, a tertiary medical center in New Haven, Connecticut, with 16 patients referred with localized cutaneous injection-site reactions from January 20 through February 12, 2021.
Main outcomes and measures: We collected each patient's demographic information, a brief relevant medical history, clinical course, and treatment (if any); and considered the findings of a histopathologic examination of 1 skin biopsy specimen.
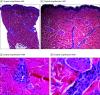
Results: Of 16 patients (median [range] age, 38 [25-89] years; 13 [81%] women), 14 patients self-identified as White and 2 as Asian. The delayed localized cutaneous reactions developed in a median (range) of 7 (2-12) days after receiving the Moderna COVID-19 vaccine. These reactions occurred at or near the injection site and were described as pruritic, painful, and edematous pink plaques. None of the participants had received the Pfizer-BioNTech vaccine. Results of a skin biopsy specimen demonstrated a mild predominantly perivascular mixed infiltrate with lymphocytes and eosinophils, consistent with a dermal hypersensitivity reaction. Of participants who had a reaction to first vaccine dose (15 of 16 patients), most (11 patients) developed a similar localized injection-site reaction to the second vaccine dose; most (10 patients) also developed the second reaction sooner as compared with the first-dose reaction.
Conclusions and relevance: Clinical and histopathologic findings of this case series study indicate that the localized injection-site reactions to the Moderna COVID-19 vaccine are a delayed hypersensitivity reaction. These reactions may occur sooner after the second dose, but they are self-limited and not associated with serious vaccine adverse effects. In contrast to immediate hypersensitivity reactions (eg, anaphylaxis, urticaria), these delayed reactions (dubbed "COVID arm") are not a contraindication to subsequent vaccination.
Conflict of interest statement
Figures

References
-
- Centers for Disease Control and Prevention . COVID Data Tracker. Accessed February 12, 2021. http://covid.cdc.gov/covid-data-tracker/#global-counts-rates
-
- Centers for Disease Control and Prevention . Local reactions, systemic reactions, adverse events, and serious adverse events: Moderna COVID-19 vaccine. Accessed February 12, 2021. https://www.cdc.gov/vaccines/covid-19/info-by-product/moderna/reactogeni...
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

