Ferroptosis in infection, inflammation, and immunity
- PMID: 33978684
- PMCID: PMC8126980
- DOI: 10.1084/jem.20210518
Ferroptosis in infection, inflammation, and immunity
Abstract
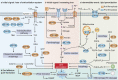
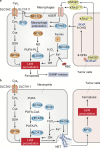
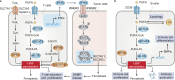
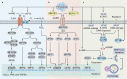
Ferroptosis is a type of regulated necrosis that is triggered by a combination of iron toxicity, lipid peroxidation, and plasma membrane damage. The upstream inducers of ferroptosis can be divided into two categories (biological versus chemical) and activate two major pathways (the extrinsic/transporter versus the intrinsic/enzymatic pathways). Excessive or deficient ferroptotic cell death is implicated in a growing list of physiological and pathophysiological processes, coupled to a dysregulated immune response. This review focuses on new discoveries related to how ferroptotic cells and their spilled contents shape innate and adaptive immunity in health and disease. Understanding the immunological characteristics and activity of ferroptotic death not only illuminates an intersection between cell death and immunity but may also lead to the development of novel treatment approaches for immunopathological diseases.
© 2021 Chen et al.
Conflict of interest statement
Disclosures: G. Kroemer reported grants from Daiichi Sankyo, Eleor, Kaleido, Lytix Pharma, PharmaMar, Samsara, Sanofi, Vascage, and Vasculox outside the submitted work; additionally, G. Kroemer is on the Board of Directors of the Bristol Myers Squibb Foundation France and is a scientific co-founder of everImmune, Samsara Therapeutics, and Therafast Bio. No other disclosures were reported.
Figures
References
-
- Amaral, E.P., Conceição E.L., Costa D.L., Rocha M.S., Marinho J.M., Cordeiro-Santos M., D’Império-Lima M.R., Barbosa T., Sher A., and Andrade B.B.. 2016. N-acetyl-cysteine exhibits potent anti-mycobacterial activity in addition to its known anti-oxidative functions. BMC Microbiol. 16:251. 10.1186/s12866-016-0872-7 - DOI - PMC - PubMed
-
- Amaral, E.P., Costa D.L., Namasivayam S., Riteau N., Kamenyeva O., Mittereder L., Mayer-Barber K.D., Andrade B.B., and Sher A.. 2019. A major role for ferroptosis in Mycobacterium tuberculosis-induced cell death and tissue necrosis. J. Exp. Med. 216:556–570. 10.1084/jem.20181776 - DOI - PMC - PubMed
-
- Anthonymuthu, T.S., Tyurina Y.Y., Sun W.Y., Mikulska-Ruminska K., Shrivastava I.H., Tyurin V.A., Cinemre F.B., Dar H.H., VanDemark A.P., Holman T.R., et al. 2021. Resolving the paradox of ferroptotic cell death: Ferrostatin-1 binds to 15LOX/PEBP1 complex, suppresses generation of peroxidized ETE-PE, and protects against ferroptosis. Redox Biol. 38:101744. 10.1016/j.redox.2020.101744 - DOI - PMC - PubMed
-
- Baba, Y., Higa J.K., Shimada B.K., Horiuchi K.M., Suhara T., Kobayashi M., Woo J.D., Aoyagi H., Marh K.S., Kitaoka H., and Matsui T.. 2018. Protective effects of the mechanistic target of rapamycin against excess iron and ferroptosis in cardiomyocytes. Am. J. Physiol. Heart Circ. Physiol. 314:H659–H668. 10.1152/ajpheart.00452.2017 - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

