Intrathoracic vs Cervical Anastomosis After Totally or Hybrid Minimally Invasive Esophagectomy for Esophageal Cancer: A Randomized Clinical Trial
- PMID: 33978698
- PMCID: PMC8117060
- DOI: 10.1001/jamasurg.2021.1555
Intrathoracic vs Cervical Anastomosis After Totally or Hybrid Minimally Invasive Esophagectomy for Esophageal Cancer: A Randomized Clinical Trial
Abstract
Background: Transthoracic minimally invasive esophagectomy (MIE) is increasingly performed as part of curative multimodality treatment. There appears to be no robust evidence on the preferred location of the anastomosis after transthoracic MIE.
Objective: To compare an intrathoracic with a cervical anastomosis in a randomized clinical trial.
Design, setting, and participants: This open, multicenter randomized clinical superiority trial was performed at 9 Dutch high-volume hospitals. Patients with midesophageal to distal esophageal or gastroesophageal junction cancer planned for curative resection were included. Data collection occurred from April 2016 through February 2020.
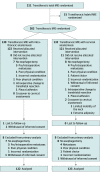
Intervention: Patients were randomly assigned (1:1) to transthoracic MIE with intrathoracic or cervical anastomosis.
Main outcomes and measures: The primary end point was anastomotic leakage requiring endoscopic, radiologic, or surgical intervention. Secondary outcomes were overall anastomotic leak rate, other postoperative complications, length of stay, mortality, and quality of life.
Results: Two hundred sixty-two patients were randomized, and 245 were eligible for analysis. Anastomotic leakage necessitating reintervention occurred in 15 of 122 patients with intrathoracic anastomosis (12.3%) and in 39 of 123 patients with cervical anastomosis (31.7%; risk difference, -19.4% [95% CI, -29.5% to -9.3%]). Overall anastomotic leak rate was 12.3% in the intrathoracic anastomosis group and 34.1% in the cervical anastomosis group (risk difference, -21.9% [95% CI, -32.1% to -11.6%]). Intensive care unit length of stay, mortality rates, and overall quality of life were comparable between groups, but intrathoracic anastomosis was associated with fewer severe complications (risk difference, -11.3% [-20.4% to -2.2%]), lower incidence of recurrent laryngeal nerve palsy (risk difference, -7.3% [95% CI, -12.1% to -2.5%]), and better quality of life in 3 subdomains (mean differences: dysphagia, -12.2 [95% CI, -19.6 to -4.7]; problems of choking when swallowing, -10.3 [95% CI, -16.4 to 4.2]; trouble with talking, -15.3 [95% CI, -22.9 to -7.7]).
Conclusions and relevance: In this randomized clinical trial, intrathoracic anastomosis resulted in better outcome for patients treated with transthoracic MIE for midesophageal to distal esophageal or gastroesophageal junction cancer.
Trial registration: Trialregister.nl Identifier: NL4183 (NTR4333).
Conflict of interest statement
Figures
Comment in
-
Does the Location Matter for the Anastomosis for Minimally Invasive Esophagectomy?JAMA Surg. 2021 Jul 1;156(7):610. doi: 10.1001/jamasurg.2021.1556. JAMA Surg. 2021. PMID: 33978688 No abstract available.
-
Generalizability of the Results and Concerns About Leakage Rates of the ICAN Trial-Reply.JAMA Surg. 2022 Feb 1;157(2):176-177. doi: 10.1001/jamasurg.2021.5266. JAMA Surg. 2022. PMID: 34668934 No abstract available.
-
Generalizability of the Results and Concerns About Leakage Rates of the ICAN Trial.JAMA Surg. 2022 Feb 1;157(2):176. doi: 10.1001/jamasurg.2021.5263. JAMA Surg. 2022. PMID: 34668941 No abstract available.
-
Generalizability of the Results and Concerns About Leakage Rates of the ICAN Trial.JAMA Surg. 2022 Feb 1;157(2):175-176. doi: 10.1001/jamasurg.2021.5260. JAMA Surg. 2022. PMID: 34668961 No abstract available.
References
-
- Al-Batran SE, Homann N, Pauligk C, et al. ; FLOT4-AIO Investigators . Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): a randomised, phase 2/3 trial. Lancet. 2019;393(10184):1948-1957. doi: 10.1016/S0140-6736(18)32557-1 - DOI - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous