The atypical antipsychotic risperidone targets hypothalamic melanocortin 4 receptors to cause weight gain
- PMID: 33978701
- PMCID: PMC8126977
- DOI: 10.1084/jem.20202484
The atypical antipsychotic risperidone targets hypothalamic melanocortin 4 receptors to cause weight gain
Abstract
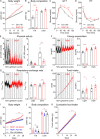
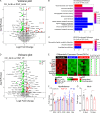
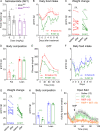
Atypical antipsychotics such as risperidone cause drug-induced metabolic syndrome. However, the underlying mechanisms remain largely unknown. Here, we report a new mouse model that reliably reproduces risperidone-induced weight gain, adiposity, and glucose intolerance. We found that risperidone treatment acutely altered energy balance in C57BL/6 mice and that hyperphagia accounted for most of the weight gain. Transcriptomic analyses in the hypothalamus of risperidone-fed mice revealed that risperidone treatment reduced the expression of Mc4r. Furthermore, Mc4r in Sim1 neurons was necessary for risperidone-induced hyperphagia and weight gain. Moreover, we found that the same pathway underlies the obesogenic effect of olanzapine-another commonly prescribed antipsychotic drug. Remarkably, whole-cell patch-clamp recording demonstrated that risperidone acutely inhibited the activity of hypothalamic Mc4r neurons via the opening of a postsynaptic potassium conductance. Finally, we showed that treatment with setmelanotide, an MC4R-specific agonist, mitigated hyperphagia and obesity in both risperidone- and olanzapine-fed mice.
© 2021 Li et al.
Conflict of interest statement
Disclosures: The authors declare no competing interests exist.
Figures





References
-
- Chen, K.Y., Muniyappa R., Abel B.S., Mullins K.P., Staker P., Brychta R.J., Zhao X., Ring M., Psota T.L., Cone R.D., et al. 2015. RM-493, a melanocortin-4 receptor (MC4R) agonist, increases resting energy expenditure in obese individuals. J. Clin. Endocrinol. Metab. 100:1639–1645. 10.1210/jc.2014-4024 - DOI - PMC - PubMed
-
- Collet, T.H., Dubern B., Mokrosinski J., Connors H., Keogh J.M., Mendes de Oliveira E., Henning E., Poitou-Bernert C., Oppert J.M., Tounian P., et al. 2017. Evaluation of a melanocortin-4 receptor (MC4R) agonist (Setmelanotide) in MC4R deficiency. Mol. Metab. 6:1321–1329. 10.1016/j.molmet.2017.06.015 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

