Contrast-enhanced ultrasound of transplant organs - liver and kidney - in children
- PMID: 33978794
- PMCID: PMC8865443
- DOI: 10.1007/s00247-020-04867-y
Contrast-enhanced ultrasound of transplant organs - liver and kidney - in children
Abstract
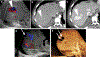
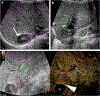
Ultrasound (US) is the first-line imaging tool for evaluating liver and kidney transplants during and after the surgical procedures. In most patients after organ transplantation, gray-scale US coupled with color/power and spectral Doppler techniques is used to evaluate the transplant organs, assess the patency of vascular structures, and identify potential complications. In technically difficult or inconclusive cases, however, contrast-enhanced ultrasound (CEUS) can provide prompt and accurate diagnostic information that is essential for management decisions. CEUS is indicated to evaluate for vascular complications including vascular stenosis or thrombosis, active bleeding, pseudoaneurysms and arteriovenous fistulas. Parenchymal indications for CEUS include evaluation for perfusion defects and focal inflammatory and non-inflammatory lesions. When transplant rejection is suspected, CEUS can assist with prompt intervention by excluding potential underlying causes for organ dysfunction. Intracavitary CEUS applications can evaluate the biliary tract of a liver transplant (e.g., for biliary strictures, bile leak or intraductal stones) or the urinary tract of a renal transplant (e.g., for urinary obstruction, urine leak or vesicoureteral reflux) as well as the position and patency of hepatic, biliary and renal drains and catheters. The aim of this review is to present current experience regarding the use of CEUS to evaluate liver and renal transplants, focusing on the examination technique and interpretation of the main imaging findings, predominantly those related to vascular complications.
Keywords: Children; Complications; Contrast-enhanced ultrasound; Intracavitary; Intravenous; Kidney; Liver; Transplant; Ultrasound; Ultrasound contrast agents.
© 2020. Springer-Verlag GmbH Germany, part of Springer Nature.
Conflict of interest statement
Figures

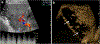
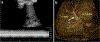




Similar articles
-
Initial experience with contrast-enhanced ultrasound in the first week after liver transplantation in children: a useful adjunct to Doppler ultrasound.Pediatr Radiol. 2021 Feb;51(2):248-256. doi: 10.1007/s00247-020-04811-0. Epub 2020 Aug 23. Pediatr Radiol. 2021. PMID: 32829424
-
Vascular rejection in renal transplant: Diagnostic value of contrast-enhanced ultrasound (CEUS) compared to biopsy.Clin Hemorheol Microcirc. 2018;69(1-2):77-82. doi: 10.3233/CH-189115. Clin Hemorheol Microcirc. 2018. PMID: 29630540
-
A Milestone: Approval of CEUS for Diagnostic Liver Imaging in Adults and Children in the USA.Ultraschall Med. 2016 Jun;37(3):229-32. doi: 10.1055/s-0042-107411. Epub 2016 Jun 8. Ultraschall Med. 2016. PMID: 27276056 English.
-
Contrast-enhanced ultrasound applications in liver transplant imaging.Abdom Radiol (NY). 2021 Jan;46(1):84-95. doi: 10.1007/s00261-020-02402-z. Abdom Radiol (NY). 2021. PMID: 31925494 Review.
-
Contrast-enhanced US in Renal Transplant Complications: Overview and Imaging Features.Radiographics. 2024 Jun;44(6):e230182. doi: 10.1148/rg.230182. Radiographics. 2024. PMID: 38781089 Review.
Cited by
-
European Society of Pediatric Radiology survey of perioperative imaging in pediatric liver transplantation: (2) intraoperative imaging.Pediatr Radiol. 2024 Feb;54(2):269-275. doi: 10.1007/s00247-023-05840-1. Epub 2024 Jan 13. Pediatr Radiol. 2024. PMID: 38216682 Free PMC article.
-
Evaluation value of contrast enhanced ultrasound quantitative parameters in ischemic-type biliary lesions after liver transplantation-a prospectively study.Abdom Radiol (NY). 2025 Aug;50(8):3497-3505. doi: 10.1007/s00261-024-04761-3. Epub 2025 Jan 25. Abdom Radiol (NY). 2025. PMID: 39862289
-
Expanding Role of Contrast-Enhanced Ultrasound and Elastography in the Evaluation of Abdominal Pathologies in Children.Diagnostics (Basel). 2025 Jul 1;15(13):1680. doi: 10.3390/diagnostics15131680. Diagnostics (Basel). 2025. PMID: 40647679 Free PMC article. Review.
-
[Imaging after kidney transplantation in childhood and adolescence].Radiologie (Heidelb). 2024 Jan;64(1):45-53. doi: 10.1007/s00117-023-01249-x. Epub 2024 Jan 5. Radiologie (Heidelb). 2024. PMID: 38180539 Review. German.
-
Contrast-enhanced US and contrast-enhanced CT for diagnosis of focal liver lesions in liver transplant recipients: A comparative study.ILIVER. 2025 Feb 13;4(1):100147. doi: 10.1016/j.iliver.2025.100147. eCollection 2025 Mar. ILIVER. 2025. PMID: 40636785 Free PMC article.
References
-
- Nixon JN, Biyyam DR, Stanescu L et al. (2013) Imaging of pediatric renal transplants and their complications: a pictorial review. Radiographics 33:1227–1251 - PubMed
-
- Friedewald SM, Molmenti EP, Friedewald JJ et al. (2005) Vascular and nonvascular complications of renal transplants: sonographic evaluation and correlation with other imaging modalities, surgery, and pathology. J Clin Ultrasound 33:127–139 - PubMed
-
- Camacho JC, Coursey-Moreno C, Telleria JC et al. (2015) Nonvascular post-liver transplantation complications: from US screening to cross-sectional and interventional imaging. Radiographics 35:87–104 - PubMed
-
- Kim N, Juarez R, Levy AD (2018) Imaging non-vascular complications of renal transplantation. Abdom Radiol 43:2555–2563 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

