Investigation of synovial fluid lubricants and inflammatory cytokines in the horse: a comparison of recombinant equine interleukin 1 beta-induced synovitis and joint lavage models
- PMID: 33980227
- PMCID: PMC8117281
- DOI: 10.1186/s12917-021-02873-2
Investigation of synovial fluid lubricants and inflammatory cytokines in the horse: a comparison of recombinant equine interleukin 1 beta-induced synovitis and joint lavage models
Abstract
Background: Lameness is a debilitating condition in equine athletes that leads to more performance limitation and loss of use than any other medical condition. There are a limited number of non-terminal experimental models that can be used to study early inflammatory and synovial fluid biophysical changes that occur in the equine joint. Here, we compare the well-established carpal IL-1β-induced synovitis model to a tarsal intra-articular lavage model, focusing on serial changes in synovial fluid inflammatory cytokines/chemokines and the synovial fluid lubricating molecules lubricin/proteoglycan 4 and hyaluronic acid. The objectives of this study were to evaluate clinical signs; synovial membrane and synovial fluid inflammation; and synovial fluid lubricants and biophysical properties in response to carpal IL-1β synovitis and tarsal intra-articular lavage.
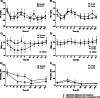
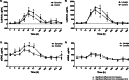
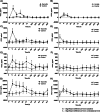
Results: Hyaluronic acid (HA) concentrations, especially high molecular weight HA, and synovial fluid viscosity decreased after both synovitis and lavage interventions. Synovial fluid lubricin concentrations increased 17-20-fold for both synovitis and lavage models, with similar changes in both affected and contralateral joints, suggesting that repeated arthrocentesis alone resulted in elevated synovial fluid lubricin concentrations. Synovitis resulted in a more severe inflammatory response based on clinical signs (temperature, heart rate, respiratory rate, lameness and joint effusion) and clinicopathological and biochemical parameters (white blood cell count, total protein, prostaglandin E2, sulfated glycosaminoglycans, tumor necrosis factor-α and CC chemokine ligands - 2, - 3, - 5 and - 11) as compared to lavage.
Conclusions: Synovial fluid lubricin increased in response to IL-1β synovitis and joint lavage but also as a result of repeated arthrocentesis. Frequent repeated arthrocentesis is associated with inflammatory changes, including increased sulfated glycosaminoglycan concentrations and decreased hyaluronic acid concentrations. Synovitis results in more significant inflammatory changes than joint lavage. Our data suggests that synovial fluid lubricin, TNF-α, CCL2, CCL3, CCL5, CCL11 and sGAG may be useful biomarkers for synovitis and post-lavage joint inflammation. Caution should be exercised when performing repeated arthrocentesis clinically or in experimental studies due to the inflammatory response and loss of HA and synovial fluid viscosity.
Keywords: Chemokine; Hyaluronic acid; Lubrication; Lubricin; Osteoarthritis; Repeated arthrocentesis; Rheology.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


Similar articles
-
Evaluation of the inflammatory response in experimentally induced synovitis in the horse: a comparison of recombinant equine interleukin 1 beta and lipopolysaccharide.Osteoarthritis Cartilage. 2012 Dec;20(12):1583-90. doi: 10.1016/j.joca.2012.08.008. Epub 2012 Aug 21. Osteoarthritis Cartilage. 2012. PMID: 22917743
-
Single injection of intra-articular autologous protein solution in horses with acute interleukin-1B-induced synovitis decreases joint pathology scores.Equine Vet J. 2025 May;57(3):806-816. doi: 10.1111/evj.14203. Epub 2024 Jul 25. Equine Vet J. 2025. PMID: 39051479 Free PMC article.
-
Effect of tumor necrosis factor antibody on synovial fluid cytokine activities in equine antebrachiocarpal joints injected with endotoxin.Am J Vet Res. 1995 Oct;56(10):1292-9. Am J Vet Res. 1995. PMID: 8928945
-
Improved Understanding of the Inflammatory Response in Synovial Fluid and Serum after Traumatic Knee Injury, Excluding Fractures of the Knee: A Systematic Review.Cartilage. 2023 Jun;14(2):198-209. doi: 10.1177/19476035221141417. Epub 2023 Jan 20. Cartilage. 2023. PMID: 36661182 Free PMC article.
-
Review of intra-articular local anaesthetic administration in horses: Clinical indications, cytotoxicity, and outcomes.Equine Vet J. 2024 Sep;56(5):870-883. doi: 10.1111/evj.14027. Epub 2023 Nov 8. Equine Vet J. 2024. PMID: 37940372 Review.
Cited by
-
Selected cytokine and chemokine concentrations in equine autologous conditioned serum are similar under defined and practically relevant storage conditions.Front Vet Sci. 2025 May 27;12:1588240. doi: 10.3389/fvets.2025.1588240. eCollection 2025. Front Vet Sci. 2025. PMID: 40496923 Free PMC article.
-
Energy metabolism dysfunction and therapeutic strategies for treating temporomandibular disorders.Front Med (Lausanne). 2025 Jun 26;12:1581446. doi: 10.3389/fmed.2025.1581446. eCollection 2025. Front Med (Lausanne). 2025. PMID: 40641978 Free PMC article. Review.
-
Articular Cartilage Regeneration by Hyaline Chondrocytes: A Case Study in Equine Model and Outcomes.Biomedicines. 2023 May 31;11(6):1602. doi: 10.3390/biomedicines11061602. Biomedicines. 2023. PMID: 37371697 Free PMC article.
-
Influence of dietary Saccharomyces cerevisiae fermentation product on markers of inflammation and cartilage metabolism in young exercising horses challenged with intra-articular lipopolysaccharide.Transl Anim Sci. 2025 Apr 28;9:txaf042. doi: 10.1093/tas/txaf042. eCollection 2025. Transl Anim Sci. 2025. PMID: 40336821 Free PMC article.
-
Targeted Analysis of the Size Distribution of Heavy Chain-Modified Hyaluronan with Solid-State Nanopores.Anal Chem. 2024 Jan 30;96(4):1606-1613. doi: 10.1021/acs.analchem.3c04387. Epub 2024 Jan 12. Anal Chem. 2024. PMID: 38215004 Free PMC article.
References
-
- Machado TSL, Massoco CO, Silva LCLC, Fülber J, Moreira JJ, Baccarin RYA. Effects of blood-derived products and sodium hyaluronate on equine synovial fluid cells and on synovial fluid from osteochondrotic joints of horses after arthroscopy and administration of treatment. Am J Vet Res. 2019;80(7):646–656. doi: 10.2460/ajvr.80.7.646. - DOI - PubMed
-
- Spadari A, Rinnovati R, Babbini S, Romagnoli N. Clinical evaluation of intra-articular administration of stanozolol to manage lameness associated with acute and chronic osteoarthritis in horses. J Equine Vet Sci. 2015;35(2):105–110. doi: 10.1016/j.jevs.2014.12.003. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

