Impact of in-hospital discontinuation with angiotensin receptor blockers or converting enzyme inhibitors on mortality of COVID-19 patients: a retrospective cohort study
- PMID: 33980231
- PMCID: PMC8114973
- DOI: 10.1186/s12916-021-01992-9
Impact of in-hospital discontinuation with angiotensin receptor blockers or converting enzyme inhibitors on mortality of COVID-19 patients: a retrospective cohort study
Abstract
Background: In the first wave of the COVID-19 pandemic, the hypothesis that angiotensin receptor blockers (ARBs) and angiotensin-converting enzyme inhibitors (ACEIs) increased the risk and/or severity of the disease was widely spread. Consequently, in many hospitals, these drugs were discontinued as a "precautionary measure". We aimed to assess whether the in-hospital discontinuation of ARBs or ACEIs, in real-life conditions, was associated with a reduced risk of death as compared to their continuation and also to compare head-to-head the continuation of ARBs with the continuation of ACEIs.
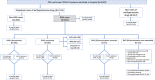
Methods: Adult patients with a PCR-confirmed diagnosis of COVID-19 requiring admission during March 2020 were consecutively selected from 7 hospitals in Madrid, Spain. Among them, we identified outpatient users of ACEIs/ARBs and divided them in two cohorts depending on treatment discontinuation/continuation at admission. Then, they were followed-up until discharge or in-hospital death. An intention-to-treat survival analysis was carried out and hazard ratios (HRs), and their 95%CIs were computed through a Cox regression model adjusted for propensity scores of discontinuation and controlled by potential mediators.
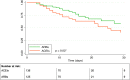
Results: Out of 625 ACEI/ARB users, 340 (54.4%) discontinued treatment. The in-hospital mortality rates were 27.6% and 27.7% in discontinuation and continuation cohorts, respectively (HR=1.01; 95%CI 0.70-1.46). No difference in mortality was observed between ARB and ACEI discontinuation (28.6% vs. 27.1%, respectively), while a significantly lower mortality rate was found among patients who continued with ARBs (20.8%, N=125) as compared to those who continued with ACEIs (33.1%, N=136; p=0.03). The head-to-head comparison (ARB vs. ACEI continuation) yielded an adjusted HR of 0.52 (95%CI 0.29-0.93), being especially notorious among males (HR=0.34; 95%CI 0.12-0.93), subjects older than 74 years (HR=0.46; 95%CI 0.25-0.85), and patients with obesity (HR=0.22; 95%CI 0.05-0.94), diabetes (HR=0.36; 95%CI 0.13-0.97), and heart failure (HR=0.12; 95%CI 0.03-0.97).
Conclusions: The discontinuation of ACEIs/ARBs at admission did not improve the in-hospital survival. On the contrary, the continuation with ARBs was associated with a trend to a reduced mortality as compared to their discontinuation and to a significantly lower mortality risk as compared to the continuation with ACEIs, particularly in high-risk patients.
Keywords: Angiotensin receptor blockers; Angiotensin-converting enzyme inhibitors; COVID-19; In-hospital treatment; Mortality; Renin-angiotensin system inhibitors.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


Comment in
-
Renin-angiotensin system blockers and COVID-19.BMC Med. 2021 Jun 4;19(1):136. doi: 10.1186/s12916-021-02012-6. BMC Med. 2021. PMID: 34082752 Free PMC article. No abstract available.
References
-
- O´Mara G. Could ACE inhibitors and particularly ARBs increase susceptibility to COVID-19 infection? BMJ. 2020;368 Available in https://www.bmj.com/content/368/bmj.m406/rr-13.
-
- Sommerstein R. Preventing a COVID-19 pandemic: ACE inhibitors as potential risk factor for fatal Covid-19. BMJ. 2020;368:m810. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

