The inhibitory effect of silencing CDCA3 on migration and proliferation in bladder urothelial carcinoma
- PMID: 33980246
- PMCID: PMC8114508
- DOI: 10.1186/s12935-021-01969-x
The inhibitory effect of silencing CDCA3 on migration and proliferation in bladder urothelial carcinoma
Abstract
Background: CDCA3 is an important component of the E3 ligase complex with SKP1 and CUL1, which could regulate the progress of cell mitosis. CDCA3 has been widely identified as a proto-oncogene in multiple human cancers, however, its role in promoting human bladder urothelial carcinoma has not been fully elucidated.
Methods: Bioinformatic methods were used to analyze the expression level of CDCA3 in human bladder urothelial carcinoma tissues and the relationship between its expression level and key clinical characteristics. In vitro studies were performed to validate the specific functions of CDCA3 in regulating cell proliferation, cell migration and cell cycle process. Alterations of related proteins was investigated by western blot assays. In vivo studies were constructed to validate whether silencing CDCA3 could inhibit the proliferation rate in mice model.
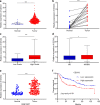
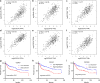
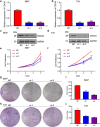
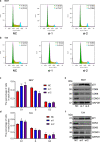
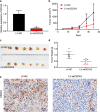
Results: Bioinformatic analysis revealed that CDCA3 was significantly up-regulated in bladder urothelial carcinoma samples and was related to key clinical characteristics, such as tumor grade and metastasis. Moreover, patients who had higher expression level of CDCA3 tend to show a shorter life span. In vitro studies revealed that silencing CDCA3 could impair the migration ability of tumor cells via down-regulating EMT-related proteins such as MMP9 and Vimentin and inhibit tumor cell growth via arresting cells in the G1 cell cycle phase through regulating cell cycle related proteins like p21. In vivo study confirmed that silencing CDCA3 could inhibit the proliferation of bladder urothelial carcinoma cells.
Conclusions: CDCA3 is an important oncogene that could strengthen the migration ability of bladder urothelial carcinoma cells and accelerate tumor cell growth via regulating cell cycle progress and is a potential biomarker of bladder urothelial carcinoma.
Keywords: Bladder urothelial carcinoma; CDCA3; Migration; cell cycle; p21.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
-
- Fairey AS, Jacobsen NE, Chetner MP, Mador DR, Metcalfe JB, Moore RB, Rourke KF, Todd GT, Venner PM, Voaklander DC, et al. Associations between comorbidity, and overall survival and bladder cancer specific survival after radical cystectomy: results from the Alberta Urology Institute Radical Cystectomy database. J Urol. 2009;182(1):85–92. doi: 10.1016/j.juro.2008.11.111. - DOI - PubMed
Grants and funding
- 31900902/National Natural Science Foundation of China
- 81772730/National Natural Science Foundation of China
- TFLC2018002/Medical Science Advancement Program (Clinical Medicine) of Wuhan University
- ZLYNXM202006/Improvement Project for Theranostic Ability on Difficulty Miscellaneous Disease (Tumor) from National Health Commission of China
- 2018ACA159/Science and Technology Department of Hubei Province Key Project
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

