ATF5 and HIF1α cooperatively activate HIF1 signaling pathway in esophageal cancer
- PMID: 33980247
- PMCID: PMC8117505
- DOI: 10.1186/s12964-021-00734-x
ATF5 and HIF1α cooperatively activate HIF1 signaling pathway in esophageal cancer
Abstract
Background: Esophageal cancer (ESCA) is one of the most common cancers worldwide and has a very poor prognosis. Hypoxia-inducible factor 1 (HIF1) signaling pathway plays a critical role in tumorigenesis and is therefore considered a potential therapeutic target in the treatment of many cancers. Activating transcription factor 5 (ATF5) facilitates the expression of various genes and has been extensively studied for its potential role in cancer treatment.
Methods: The expression level of ATF5 in clinic sample was detected by quantitative real time PCR and immunohistochemistry. ATF5 biological function was investigated by western blot, cell cycle analysis, cell viability assay, luciferase reporter assays, colony formation assay, transwell assay, wound healing assay, tube formation assay, and ELISA assay. CHIP and Re-CHIP assay, GST-pulldown, and RNA-sequencing were used to study the cross-talks between ATF5 and HIF1 complex. Mouse xenograft study was utilized to study the correlation of ATF5 and tumor growth in vivo. Student's t-test or Chi-square test was used for statistical analysis.
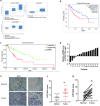
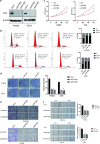
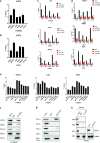
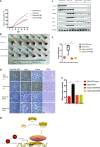
Results: Here, we first found ATF5 was dramatically upregulated in ESCA cancer and related with poor survival time. Next, we found that the expression level of ATF5 had a positive relationship with the proliferation, migration, and invasion ability of ESCA cells. Besides, we innovatively found that ATF5 functions as a novel coactivator in HIF1 transcription complex by binding to HIF1α. Further, we demonstrated that silencing ATF5 phenocopies HIF1α knockdown in tumorigenic properties in vitro and inhibited ESCA tumor angiogenesis and proliferation in vivo.
Conclusion: Herein, we found ATF5 as a novel component of the HIF1 transcription complex. The findings of the present study may provide new insights into the development of a novel and more efficient therapeutic strategy against ESCA. Video abstract.
Keywords: ATF5; Esophageal cancer; HIF1.
Conflict of interest statement
There was no conflict of interest.
Figures



Similar articles
-
Increased expression of EIF5A2, via hypoxia or gene amplification, contributes to metastasis and angiogenesis of esophageal squamous cell carcinoma.Gastroenterology. 2014 Jun;146(7):1701-13.e9. doi: 10.1053/j.gastro.2014.02.029. Epub 2014 Feb 21. Gastroenterology. 2014. PMID: 24561231
-
Histone demethylase KDM4A plays an oncogenic role in nasopharyngeal carcinoma by promoting cell migration and invasion.Exp Mol Med. 2021 Aug;53(8):1207-1217. doi: 10.1038/s12276-021-00657-0. Epub 2021 Aug 12. Exp Mol Med. 2021. PMID: 34385569 Free PMC article.
-
FSTL3 promotes colorectal cancer by activating the HIF1 pathway.Gene. 2025 Jun 20;954:149435. doi: 10.1016/j.gene.2025.149435. Epub 2025 Mar 26. Gene. 2025. PMID: 40154584
-
Impact of miRNAs involved in the STAT3 signaling pathway on esophageal cancer (Review).Oncol Rep. 2025 Feb;53(2):27. doi: 10.3892/or.2024.8860. Epub 2025 Jan 3. Oncol Rep. 2025. PMID: 39749694 Review.
-
The transcription factor ATF5: role in neurodevelopment and neural tumors.J Neurochem. 2009 Jan;108(1):11-22. doi: 10.1111/j.1471-4159.2008.05749.x. Epub 2008 Nov 15. J Neurochem. 2009. PMID: 19046351 Free PMC article. Review.
Cited by
-
Targeting Transcription Factors ATF5, CEBPB and CEBPD with Cell-Penetrating Peptides to Treat Brain and Other Cancers.Cells. 2023 Feb 11;12(4):581. doi: 10.3390/cells12040581. Cells. 2023. PMID: 36831248 Free PMC article. Review.
-
A bird's eye view of mitochondrial unfolded protein response in cancer: mechanisms, progression and further applications.Cell Death Dis. 2024 Sep 11;15(9):667. doi: 10.1038/s41419-024-07049-y. Cell Death Dis. 2024. PMID: 39261452 Free PMC article. Review.
-
Single-cell transcriptome analysis of tumor immune microenvironment characteristics in colorectal cancer liver metastasis.Ann Transl Med. 2022 Nov;10(21):1170. doi: 10.21037/atm-22-5270. Ann Transl Med. 2022. PMID: 36467341 Free PMC article.
-
Advancements in Activating Transcription Factor 5 Function in Regulating Cell Stress and Survival.Int J Mol Sci. 2022 Jun 27;23(13):7129. doi: 10.3390/ijms23137129. Int J Mol Sci. 2022. PMID: 35806136 Free PMC article. Review.
-
Mitochondrial Quality Control via Mitochondrial Unfolded Protein Response (mtUPR) in Ageing and Neurodegenerative Diseases.Biomolecules. 2023 Dec 13;13(12):1789. doi: 10.3390/biom13121789. Biomolecules. 2023. PMID: 38136659 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
- 2016CFB550/Natural Science Foundation of Hubei Province
- 3011540027/Double first class construction project of Tongji Medical College of Huazhong University of science and technology--Research clinician funding scheme
- 5001540077/Double first class construction project of Tongji Medical College of Huazhong University of science and technology--Research clinician funding scheme
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

