The YAP/HIF-1α/miR-182/EGR2 axis is implicated in asthma severity through the control of Th17 cell differentiation
- PMID: 33980319
- PMCID: PMC8117288
- DOI: 10.1186/s13578-021-00560-1
The YAP/HIF-1α/miR-182/EGR2 axis is implicated in asthma severity through the control of Th17 cell differentiation
Abstract
Background: Asthma is a heterogeneous chronic inflammatory disease of the airway, involving reversible airflow limitation and airway remodeling. T helper 17 (Th17) cells play an important role in the pathogenesis of allergic asthma. However, there is limited understanding of the signaling pathways controlling Th17 cell differentiation in asthma. The aim of this study was to investigate if the Yes-associated protein (YAP)/hypoxia inducible factor-1α (HIF-1α)/microRNA-182 (miR-182)/early growth response 2 (EGR2) axis is involved in mediating Th17 cell differentiation and disease severity in asthma.
Methods: The study included 29 pediatric patients with asthma, 22 healthy volunteers, ovalbumin-induced murine asthma models, and mouse naive CD4+ T cells. The subpopulation of Th17 cells was examined by flow cytometry. The levels of interleukin-17A were determined by enzyme linked immunosorbent assay. Chromatin immunoprecipitation-quantitative polymerase chain reaction assays and dual-luciferase reporter gene assays were performed to examine interactions between HIF-1α and miR-182, and between miR-182 and EGR2.
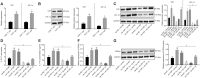
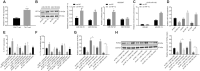
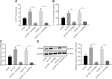
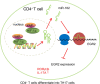
Results: YAP, HIF-1α, and miR-182 were upregulated but EGR2 was downregulated in human and mouse peripheral blood mononuclear cells from the asthma group. Abundant expression of YAP and HIF-1α promoted miR-182 expression and then inhibited EGR2, a target of miR-182, thus enhancing Th17 differentiation and deteriorating asthma and lipid metabolism dysfunction. In addition, in vivo overexpression of EGR2 countered the promoting effect of the YAP/HIF-1α/miR-182 axis on asthma and lipid metabolism dysfunction.
Conclusion: These results indicate that activation of the YAP/HIF-1α/miR-182/EGR2 axis may promote Th17 cell differentiation, exacerbate asthma development, and aggravate lipid metabolism dysfunction, thus suggesting a potential therapeutic target for asthma.
Keywords: Asthma; Differentiation; Dyslipidemia; EGR2; HIF-1α; MiR-182; Th17 cells; YAP.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures




Similar articles
-
MBD2 regulates differentiation and function of Th17 cells in neutrophils- dominant asthma via HIF-1α.J Inflamm (Lond). 2018 Aug 20;15:15. doi: 10.1186/s12950-018-0191-x. eCollection 2018. J Inflamm (Lond). 2018. PMID: 30150897 Free PMC article.
-
The PBX1/miR-141-miR-200a/EGR2/SOCS3 Axis; Integrative Analysis of Interaction Networks to Discover the Possible Mechanism of MiR-141 and MiR-200a-Mediated Th17 Cell Differentiation.Iran J Biotechnol. 2023 Jan 1;21(1):e3211. doi: 10.30498/ijb.2022.317078.3211. eCollection 2023 Jan. Iran J Biotechnol. 2023. PMID: 36811100 Free PMC article.
-
MiR-150-5p regulates EGR2 to promote the development of chronic rhinosinusitis via the DC-Th axis.Int Immunopharmacol. 2018 Jan;54:188-197. doi: 10.1016/j.intimp.2017.11.011. Epub 2017 Nov 16. Int Immunopharmacol. 2018. PMID: 29153954
-
METTL3/miR-192-5p/SCD1 Axis Regulates Lipid Metabolism to Affect T Cell Differentiation in Asthma.Mediators Inflamm. 2025 Jan 19;2025:4955849. doi: 10.1155/mi/4955849. eCollection 2025. Mediators Inflamm. 2025. PMID: 39867638 Free PMC article.
-
Relationship between Hypoxic and Immune Pathways Activation in the Progression of Neuroinflammation: Role of HIF-1α and Th17 Cells.Int J Mol Sci. 2023 Feb 4;24(4):3073. doi: 10.3390/ijms24043073. Int J Mol Sci. 2023. PMID: 36834484 Free PMC article. Review.
Cited by
-
Nur77 Mediates Anaphylaxis by Regulating miR-21a.Curr Issues Mol Biol. 2024 Apr 6;46(4):3175-3192. doi: 10.3390/cimb46040199. Curr Issues Mol Biol. 2024. PMID: 38666929 Free PMC article.
-
Lipid metabolism-related genes are involved in the occurrence of asthma and regulate the immune microenvironment.BMC Genomics. 2024 Feb 1;25(1):129. doi: 10.1186/s12864-023-09795-3. BMC Genomics. 2024. PMID: 38297226 Free PMC article.
-
YAP as a potential therapeutic target for myofibroblast formation in asthma.Respir Res. 2025 Feb 12;26(1):51. doi: 10.1186/s12931-025-03115-x. Respir Res. 2025. PMID: 39939959 Free PMC article.
-
Long-term alterations in lung epithelial cells after EL-RSV infection exacerbate allergic responses through IL-1β-induced pathways.Mucosal Immunol. 2024 Oct;17(5):1072-1088. doi: 10.1016/j.mucimm.2024.07.007. Epub 2024 Jul 27. Mucosal Immunol. 2024. PMID: 39069078 Free PMC article.
-
The Role of Yes-Associated Protein in Inflammatory Diseases and Cancer.MedComm (2020). 2025 Mar 10;6(3):e70128. doi: 10.1002/mco2.70128. eCollection 2025 Mar. MedComm (2020). 2025. PMID: 40066231 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

