Stem cell therapies and benefaction of somatic cell nuclear transfer cloning in COVID-19 era
- PMID: 33980321
- PMCID: PMC8114669
- DOI: 10.1186/s13287-021-02334-5
Stem cell therapies and benefaction of somatic cell nuclear transfer cloning in COVID-19 era
Abstract
Background: The global health emergency of COVID-19 has necessitated the development of multiple therapeutic modalities including vaccinations, antivirals, anti-inflammatory, and cytoimmunotherapies, etc. COVID-19 patients suffer from damage to various organs and vascular structures, so they present multiple health crises. Mesenchymal stem cells (MSCs) are of interest to treat acute respiratory distress syndrome (ARDS) caused by SARS-CoV-2 infection.
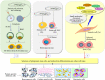
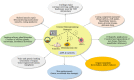
Main body: Stem cell-based therapies have been verified for prospective benefits in copious preclinical and clinical studies. MSCs confer potential benefits to develop various cell types and organoids for studying virus-human interaction, drug testing, regenerative medicine, and immunomodulatory effects in COVID-19 patients. Apart from paving the ways to augment stem cell research and therapies, somatic cell nuclear transfer (SCNT) holds unique ability for a wide range of health applications such as patient-specific or isogenic cells for regenerative medicine and breeding transgenic animals for biomedical applications. Being a potent cell genome-reprogramming tool, the SCNT has increased prominence of recombinant therapeutics and cellular medicine in the current era of COVID-19. As SCNT is used to generate patient-specific stem cells, it avoids dependence on embryos to obtain stem cells.
Conclusions: The nuclear transfer cloning, being an ideal tool to generate cloned embryos, and the embryonic stem cells will boost drug testing and cellular medicine in COVID-19.
Keywords: Bio-pharming; COVID-19; Genome reprogramming; Regenerative medicine; SARS-CoV-2; SCNT; Stem cells.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
Cloning by SCNT: Integrating Technical and Biology-Driven Advances.Methods Mol Biol. 2023;2647:1-35. doi: 10.1007/978-1-0716-3064-8_1. Methods Mol Biol. 2023. PMID: 37041327
-
Nuclear reprogramming of cloned embryos and its implications for therapeutic cloning.Nat Genet. 2007 Mar;39(3):295-302. doi: 10.1038/ng1973. Nat Genet. 2007. PMID: 17325680 Review.
-
Human therapeutic cloning (NTSC): applying research from mammalian reproductive cloning.Stem Cell Rev. 2006;2(4):265-76. doi: 10.1007/BF02698053. Stem Cell Rev. 2006. PMID: 17848713
-
Stalling SARS-CoV2 infection with stem cells: can regenerating perinatal tissue mesenchymal stem cells offer a multi-tiered therapeutic approach to COVID-19?Placenta. 2022 Jan;117:161-168. doi: 10.1016/j.placenta.2021.12.005. Epub 2021 Dec 6. Placenta. 2022. PMID: 34915433 Free PMC article. Review.
-
Double sperm cloning (DSC) is a promising strategy in mammalian genetic engineering and stem cell research.Stem Cell Res Ther. 2020 Sep 7;11(1):388. doi: 10.1186/s13287-020-01907-0. Stem Cell Res Ther. 2020. PMID: 32894201 Free PMC article. Review.
Cited by
-
Stem Cell Transplantation Therapy and Neurological Disorders: Current Status and Future Perspectives.Biology (Basel). 2022 Jan 17;11(1):147. doi: 10.3390/biology11010147. Biology (Basel). 2022. PMID: 35053145 Free PMC article. Review.
-
Effectiveness of RCTs Pooling Evidence on Mesenchymal Stem Cell (MSC) Therapeutic Applications During COVID-19 Epidemic: A Systematic Review.Biologics. 2023 May 18;17:85-112. doi: 10.2147/BTT.S404421. eCollection 2023. Biologics. 2023. PMID: 37223116 Free PMC article. Review.
-
Clinical efficacy analysis of mesenchymal stem cell therapy in patients with COVID-19: A systematic review.World J Clin Cases. 2022 Sep 26;10(27):9714-9726. doi: 10.12998/wjcc.v10.i27.9714. World J Clin Cases. 2022. PMID: 36186213 Free PMC article.
-
Revolutionary Regeneration Therapy Utilizing Dental Stem Cells and State-of-the-Art Nanotechnology Devices to Heal Injured Teeth and Tissues.Avicenna J Med Biotechnol. 2025 Apr-Jun;17(2):83-97. doi: 10.18502/ajmb.v17i2.18559. Avicenna J Med Biotechnol. 2025. PMID: 40453915 Free PMC article. Review.
-
Stem Cells as a Model of Study of SARS-CoV-2 and COVID-19: A Systematic Review of the Literature.Biomed Res Int. 2021 Aug 25;2021:9915927. doi: 10.1155/2021/9915927. eCollection 2021. Biomed Res Int. 2021. PMID: 34458372 Free PMC article.
References
-
- Bilal M, Khan MI, Nazir MS, Ahmed I, Iqbal HMN. Coronaviruses and COVID-19 – complications and lessons learned for the future. J Pure Appl Microbiol. 2020;14:725–731. doi: 10.22207/JPAM.14.SPL1.09. - DOI
-
- Hussain N, Ahmed A, Khan MI, Zhu W, Nadeem Z, Bilal M. A real-time updated portrayal of covid-19 diagnosis and therapeutic options. J Exp Biol Agric Sci. 2020;8:S21–S33.
-
- Iqbal MS, Sardar N, Akmal W, Qadri AM, Nawaz R, Miraj A, et al. Severe acute respiratory syndrome coronaviruses and 21st century pandemic: an overview of functional receptors and challenge of therapeutic success. J Exp Biol Agric Sci. 2020;8:S87–102.
-
- Shah STA, Iftikhar A, Khan MI, Mansoor M, Mirza AF, Bilal M. Predicting covid-19 infections prevalence using linear regression tool. J Exp Biol Agric Sci. 2020;8:S01–S08.
-
- Ali Shah ST, Mansoor M, Mirza AF, Dilshad M, Khan MI, Farwa R, et al. Predicting COVID-19 spread in Pakistan using the siR model. J Pure Appl Microbiol. 2020;14:1423–1430. doi: 10.22207/JPAM.14.2.40. - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

