Functional interferometric diffusing wave spectroscopy of the human brain
- PMID: 33980479
- PMCID: PMC8115931
- DOI: 10.1126/sciadv.abe0150
Functional interferometric diffusing wave spectroscopy of the human brain
Abstract
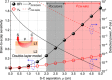
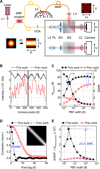
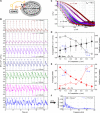
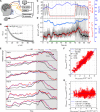
Cerebral blood flow (CBF) is essential for brain function, and CBF-related signals can inform us about brain activity. Yet currently, high-end medical instrumentation is needed to perform a CBF measurement in adult humans. Here, we describe functional interferometric diffusing wave spectroscopy (fiDWS), which introduces and collects near-infrared light via the scalp, using inexpensive detector arrays to rapidly monitor coherent light fluctuations that encode brain blood flow index (BFI), a surrogate for CBF. Compared to other functional optical approaches, fiDWS measures BFI faster and deeper while also providing continuous wave absorption signals. Achieving clear pulsatile BFI waveforms at source-collector separations of 3.5 cm, we confirm that optical BFI, not absorption, shows a graded hypercapnic response consistent with human cerebrovascular physiology, and that BFI has a better contrast-to-noise ratio than absorption during brain activation. By providing high-throughput measurements of optical BFI at low cost, fiDWS will expand access to CBF.
Copyright © 2021 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works. Distributed under a Creative Commons Attribution NonCommercial License 4.0 (CC BY-NC).
Figures


References
-
- Morotti A., Busto G., Bernardoni A., Marini S., Casetta I., Fainardi E., Association between perihematomal perfusion and intracerebral hemorrhage outcome. Neurocrit. Care 33, 525–532 (2020). - PubMed
-
- Bouma G. J., Muizelaar J. P., Cerebral blood flow, cerebral blood volume, and cerebrovascular reactivity after severe head injury. J. Neurotrauma 9 (Suppl. 1), S333–S348 (1992). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

