Enhanced interfacial electron transfer between thylakoids and RuO2 nanosheets for photosynthetic energy harvesting
- PMID: 33980487
- PMCID: PMC8115919
- DOI: 10.1126/sciadv.abf2543
Enhanced interfacial electron transfer between thylakoids and RuO2 nanosheets for photosynthetic energy harvesting
Abstract
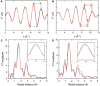
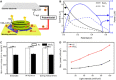
The harvesting of photosynthetic electrons (PEs) directly from photosynthetic complexes has been demonstrated over the past decade. However, their limited efficiency and stability have hampered further practical development. For example, despite its importance, the interfacial electron transfer between the photosynthetic apparatus and the electrode has received little attention. In this study, we modified electrodes with RuO2 nanosheets to enhance the extraction of PEs from thylakoids, and the PE transfer was promoted by proton adsorption and surface polarity characteristics. The adsorbed protons maintained the potential of an electrode more positive, and the surface polarity enhanced thylakoid attachment to the electrode in addition to promoting ensemble docking between the redox species and the electrode. The RuO2 bioanode exhibited a five times larger current density and a four times larger power density than the Au bioanode. Last, the electric calculators were successfully powered by photosynthetic energy using a RuO2 bioanode.
Copyright © 2021 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works. Distributed under a Creative Commons Attribution NonCommercial License 4.0 (CC BY-NC).
Figures




References
-
- Gust D., Moore T. A., Mimicking photosynthesis. Science 244, 35–41 (1989). - PubMed
-
- Ryu W., Bai S.-J., Park J. S., Huang Z., Moseley J., Fabian T., Fasching R. J., Grossman A. R., Prinz F. B., Direct extraction of photosynthetic electrons from single algal cells by nanoprobing system. Nano Lett. 10, 1137–1143 (2010). - PubMed
-
- Kim L. H., Kim Y. J., Hong H., Yang D., Han M., Yoo G., Song H. W., Chae Y., Pyun J. C., Grossman A. R., Ryu W., Patterned nanowire electrode array for direct extraction of photosynthetic electrons from multiple living algal cells. Adv. Funct. Mater. 26, 7679–7689 (2016).
-
- Hong H., Kim Y. J., Han M., Yoo G., Song H. W., Chae Y., Pyun J.-C., Grossman A. R., Ryu W., Prolonged and highly efficient intracellular extraction of photosynthetic electrons from single algal cells by optimized nanoelectrode insertion. Nano Res. 11, 397–409 (2018).
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

