Impact of early low-calorie low-protein versus standard-calorie standard-protein feeding on outcomes of ventilated adults with shock: design and conduct of a randomised, controlled, multicentre, open-label, parallel-group trial (NUTRIREA-3)
- PMID: 33980526
- PMCID: PMC8117996
- DOI: 10.1136/bmjopen-2020-045041
Impact of early low-calorie low-protein versus standard-calorie standard-protein feeding on outcomes of ventilated adults with shock: design and conduct of a randomised, controlled, multicentre, open-label, parallel-group trial (NUTRIREA-3)
Abstract
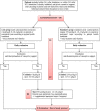
Introduction: International guidelines include early nutritional support (≤48 hour after admission), 20-25 kcal/kg/day, and 1.2-2 g/kg/day protein at the acute phase of critical illness. Recent data challenge the appropriateness of providing standard amounts of calories and protein during acute critical illness. Restricting calorie and protein intakes seemed beneficial, suggesting a role for metabolic pathways such as autophagy, a potential key mechanism in safeguarding cellular integrity, notably in the muscle, during critical illness. However, the optimal calorie and protein supply at the acute phase of severe critical illness remains unknown. NUTRIREA-3 will be the first trial to compare standard calorie and protein feeding complying with guidelines to low-calorie low-protein feeding. We hypothesised that nutritional support with calorie and protein restriction during acute critical illness decreased day 90 mortality and/or dependency on intensive care unit (ICU) management in mechanically ventilated patients receiving vasoactive amine therapy for shock, compared with standard calorie and protein targets.
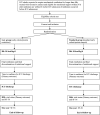
Methods and analysis: NUTRIREA-3 is a randomised, controlled, multicentre, open-label trial comparing two parallel groups of patients receiving invasive mechanical ventilation and vasoactive amine therapy for shock and given early nutritional support according to one of two strategies: early calorie-protein restriction (6 kcal/kg/day-0.2-0.4 g/kg/day) or standard calorie-protein targets (25 kcal/kg/day, 1.0-1.3 g/kg/day) at the acute phase defined as the first 7 days in the ICU. We will include 3044 patients in 61 French ICUs. Two primary end-points will be evaluated: day 90 mortality and time to ICU discharge readiness. The trial will be considered positive if significant between-group differences are found for one or both alternative primary endpoints. Secondary outcomes include hospital-acquired infections and nutritional, clinical and functional outcomes.
Ethics and dissemination: The NUTRIREA-3 study has been approved by the appropriate ethics committee. Patients are included after informed consent. Results will be submitted for publication in peer-reviewed journals.
Trial registration number: NCT03573739.
Keywords: adult intensive & critical care; clinical trials; nutrition & dietetics.
© Author(s) (or their employer(s)) 2021. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: JR had travel and accommodation expenses to attend scientific meetings covered by Baxter and Fresenius.
Figures
References
-
- Taylor BE, McClave SA, Martindale RG. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: Society of critical care medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (ASPEN). Crit Care Med 2016;44:390–438. - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical