The CD8+ T Cell Noncytotoxic Antiviral Responses
- PMID: 33980586
- PMCID: PMC8139528
- DOI: 10.1128/MMBR.00155-20
The CD8+ T Cell Noncytotoxic Antiviral Responses
Abstract
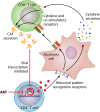
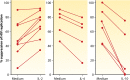
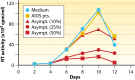
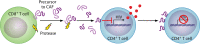
The CD8+ T cell noncytotoxic antiviral response (CNAR) was discovered during studies of asymptomatic HIV-infected subjects more than 30 years ago. In contrast to CD8+ T cell cytotoxic lymphocyte (CTL) activity, CNAR suppresses HIV replication without target cell killing. This activity has characteristics of innate immunity: it acts on all retroviruses and thus is neither epitope specific nor HLA restricted. The HIV-associated CNAR does not affect other virus families. It is mediated, at least in part, by a CD8+ T cell antiviral factor (CAF) that blocks HIV transcription. A variety of assays used to measure CNAR/CAF and the effects on other retrovirus infections are described. Notably, CD8+ T cell noncytotoxic antiviral responses have now been observed with other virus families but are mediated by different cytokines. Characterizing the protein structure of CAF has been challenging despite many biologic, immunologic, and molecular studies. It represents a low-abundance protein that may be identified by future next-generation sequencing approaches. Since CNAR/CAF is a natural noncytotoxic activity, it could provide promising strategies for HIV/AIDS therapy, cure, and prevention.
Keywords: CD8+ T cells; HIV transcription; elite controllers; human immunodeficiency virus; innate immunity; noncytotoxic antiviral activity; soluble antiviral factor.
Copyright © 2021 American Society for Microbiology.
Figures


References
-
- Migueles SA, Rogan DC, Gavil NV, Kelly EP, Toulmin SA, Wang LT, Lack J, Ward AJ, Pryal PF, Ludwig AK, Medina RG, Apple BJ, Toumanios CN, Poole AL, Rehm CA, Jones SE, Liang CJ, Connors M. 2020. Antigenic restimulation of virus-specific memory CD8+ T cells requires days of lytic protein accumulation for maximal cytotoxic capacity. J Virol 94:e01595-20. 10.1128/JVI.01595-20. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

