Endogenous and Borrowed Proteolytic Activity in the Borrelia
- PMID: 33980587
- PMCID: PMC8139524
- DOI: 10.1128/MMBR.00217-20
Endogenous and Borrowed Proteolytic Activity in the Borrelia
Abstract
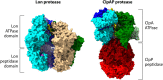
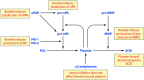
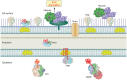
The Borrelia spp. are tick-borne pathogenic spirochetes that include the agents of Lyme disease and relapsing fever. As part of their life cycle, the spirochetes traffic between the tick vector and the vertebrate host, which requires significant physiological changes and remodeling of their outer membranes and proteome. This crucial proteome resculpting is carried out by a diverse set of proteases, adaptor proteins, and related chaperones. Despite its small genome, Borrelia burgdorferi has dedicated a large percentage of its genome to proteolysis, including a full complement of ATP-dependent proteases. Energy-driven proteolysis appears to be an important physiological feature of this dual-life-cycle bacterium. The proteolytic arsenal of Borrelia is strategically deployed for disposal of proteins no longer required as they move from one stage to another or are transferred from one host to another. Likewise, the Borrelia spp. are systemic organisms that need to break down and move through host tissues and barriers, and so their unique proteolytic resources, both endogenous and borrowed, make movement more feasible. Both the Lyme disease and relapsing fever Borrelia spp. bind plasminogen as well as numerous components of the mammalian plasminogen-activating system. This recruitment capacity endows the spirochetes with a borrowed proteolytic competency that can lead to increased invasiveness.
Keywords: Borrelia; borrowed proteolysis; plasminogen; proteases; proteolytic enzymes.
Copyright © 2021 American Society for Microbiology.
Figures

References
-
- Swellengrebel NH. 1907. De l’anaphylaxie en général. Ann Inst Pasteur 21:562–586.
-
- Fukunaga M, Takahashi Y, Tsuruta Y, Matsushita O, Ralph D, McClelland M, Nakao M. 1995. Genetic and phenotypic analysis of Borrelia miyamotoi sp. nov., isolated from the ixodid tick Ixodes persulcatus, the vector for Lyme disease in Japan. Int J Syst Bacteriol 45:804–810. doi: 10.1099/00207713-45-4-804. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

