The 5-HT3 Receptor Affects Rotavirus-Induced Motility
- PMID: 33980599
- PMCID: PMC8274622
- DOI: 10.1128/JVI.00751-21
The 5-HT3 Receptor Affects Rotavirus-Induced Motility
Abstract
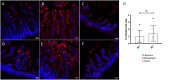
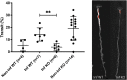
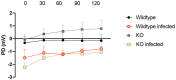
Rotavirus infection is highly prevalent in children, and the most severe effects are diarrhea and vomiting. It is well accepted that the enteric nervous system (ENS) is activated and plays an important role, but knowledge of how rotavirus activates nerves within ENS and to the vomiting center is lacking. Serotonin is released during rotavirus infection, and antagonists to the serotonin receptor subtype 3 (5-HT3 receptor) can attenuate rotavirus-induced diarrhea. In this study, we used a 5-HT3 receptor knockout (KO) mouse model to investigate the role of this receptor in rotavirus-induced diarrhea, motility, electrolyte secretion, inflammatory response, and vomiting reflex. The number of diarrhea days (P = 0.03) and the number of mice with diarrhea were lower in infected 5-HT3 receptor KO than wild-type pups. In vivo investigation of fluorescein isothiocyanate (FITC)-dextran transit time showed that intestinal motility was lower in the infected 5-HT3 receptor KO compared to wild-type mice (P = 0.0023). Ex vivo Ussing chamber measurements of potential difference across the intestinal epithelia showed no significant difference in electrolyte secretion between the two groups. Immediate early gene cFos expression level showed no difference in activation of the vomiting center in the brain. Cytokine analysis of the intestine indicated a low effect of inflammatory response in rotavirus-infected mice lacking the 5-HT3 receptor. Our findings indicate that the 5-HT3 receptor is involved in rotavirus-induced diarrhea via its effect on intestinal motility and that the vagus nerve signaling to the vomiting center occurs also in the absence of the 5-HT3 receptor. IMPORTANCE The mechanisms underlying rotavirus-induced diarrhea and vomiting are not yet fully understood. To better understand rotavirus pathophysiology, characterization of nerve signaling within the ENS and through vagal efferent nerves to the brain, which have been shown to be of great importance to the disease, is necessary. Serotonin (5-HT), a mediator of both diarrhea and vomiting, has been shown to be released from enterochromaffin cells in response to rotavirus infection and the rotavirus enterotoxin NSP4. Here, we investigated the role of the serotonin receptor 5-HT3, which is known to be involved in the nerve signals that regulate gut motility, intestinal secretion, and signal transduction through the vagus nerve to the brain. We show that the 5-HT3 receptor is involved in rotavirus-induced diarrhea by promoting intestinal motility. The findings shed light on new treatment possibilities for rotavirus diarrhea.
Keywords: 5-HT3 receptor; diarrhea; disease mechanisms; disease symptoms; motility; rotavirus; serotonin; vomiting.
Figures
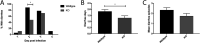


References
-
- Tate JE, Burton AH, Boschi-Pinto C, Parashar UD, World Health Organization-Coordinated Global Rotavirus Surveillance Network. 2016. Global, regional, and national estimates of rotavirus mortality in children <5 years of age, 2000–2013. Clin Infect Dis 62(Suppl 2):S96–S105. 10.1093/cid/civ1013. - DOI - PMC - PubMed
-
- Troeger C, Khalil IA, Rao PC, Cao S, Blacker BF, Ahmed T, Armah G, Bines JE, Brewer TG, Colombara DV, Kang G, Kirkpatrick BD, Kirkwood CD, Mwenda JM, Parashar UD, Petri WA, Jr., Riddle MS, Steele AD, Thompson RL, Walson JL, Sanders JW, Mokdad AH, Murray CJL, Hay SI, Reiner RC, Jr.. 2018. Rotavirus vaccination and the global burden of rotavirus diarrhea among children younger than 5 years. JAMA Pediatr 172:958–965. 10.1001/jamapediatrics.2018.1960. - DOI - PMC - PubMed
-
- Hagbom M, Istrate C, Engblom D, Karlsson T, Rodriguez-Diaz J, Buesa J, Taylor JA, Loitto VM, Magnusson KE, Ahlman H, Lundgren O, Svensson L. 2011. Rotavirus stimulates release of serotonin (5-HT) from human enterochromaffin cells and activates brain structures involved in nausea and vomiting. PLoS Pathog 7:e1002115. 10.1371/journal.ppat.1002115. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

