Public health impact of delaying second dose of BNT162b2 or mRNA-1273 covid-19 vaccine: simulation agent based modeling study
- PMID: 33980718
- PMCID: PMC8114182
- DOI: 10.1136/bmj.n1087
Public health impact of delaying second dose of BNT162b2 or mRNA-1273 covid-19 vaccine: simulation agent based modeling study
Erratum in
-
Public health impact of delaying second dose of BNT162b2 or mRNA-1273 covid-19 vaccine: simulation agent based modeling study.BMJ. 2021 May 25;373:n1334. doi: 10.1136/bmj.n1334. BMJ. 2021. PMID: 34035040 Free PMC article. No abstract available.
Abstract
Objective: To estimate population health outcomes with delayed second dose versus standard schedule of SARS-CoV-2 mRNA vaccination.
Design: Simulation agent based modeling study.
Setting: Simulated population based on real world US county.
Participants: The simulation included 100 000 agents, with a representative distribution of demographics and occupations. Networks of contacts were established to simulate potentially infectious interactions though occupation, household, and random interactions.
Interventions: Simulation of standard covid-19 vaccination versus delayed second dose vaccination prioritizing the first dose. The simulation runs were replicated 10 times. Sensitivity analyses included first dose vaccine efficacy of 50%, 60%, 70%, 80%, and 90% after day 12 post-vaccination; vaccination rate of 0.1%, 0.3%, and 1% of population per day; assuming the vaccine prevents only symptoms but not asymptomatic spread (that is, non-sterilizing vaccine); and an alternative vaccination strategy that implements delayed second dose for people under 65 years of age, but not until all those above this age have been vaccinated.
Main outcome measures: Cumulative covid-19 mortality, cumulative SARS-CoV-2 infections, and cumulative hospital admissions due to covid-19 over 180 days.
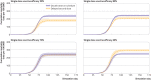
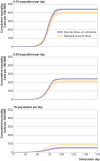
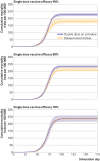
Results: Over all simulation replications, the median cumulative mortality per 100 000 for standard dosing versus delayed second dose was 226 v 179, 233 v 207, and 235 v 236 for 90%, 80%, and 70% first dose efficacy, respectively. The delayed second dose strategy was optimal for vaccine efficacies at or above 80% and vaccination rates at or below 0.3% of the population per day, under both sterilizing and non-sterilizing vaccine assumptions, resulting in absolute cumulative mortality reductions between 26 and 47 per 100 000. The delayed second dose strategy for people under 65 performed consistently well under all vaccination rates tested.
Conclusions: A delayed second dose vaccination strategy, at least for people aged under 65, could result in reduced cumulative mortality under certain conditions.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: no support from any organization for the submitted work; no financial relationships with any organizations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
Figures


References
-
- Voysey M, Clemens SAC, Madhi SA, et al. Oxford COVID Vaccine Trial Group . Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 2021;397:99-111. 10.1016/S0140-6736(20)32661-1 - DOI - PMC - PubMed
-
- A Study of Ad26.COV2.S for the Prevention of SARS-CoV-2-Mediated COVID-19 in Adult Participants (ENSEMBLE). 2021 https://clinicaltrials.gov/ct2/show/NCT04505722.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
