RNA m6A reader YTHDF2 facilitates lung adenocarcinoma cell proliferation and metastasis by targeting the AXIN1/Wnt/β-catenin signaling
- PMID: 33980824
- PMCID: PMC8116339
- DOI: 10.1038/s41419-021-03763-z
RNA m6A reader YTHDF2 facilitates lung adenocarcinoma cell proliferation and metastasis by targeting the AXIN1/Wnt/β-catenin signaling
Abstract
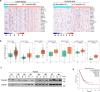
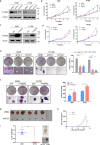
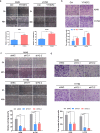
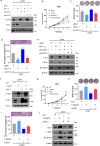
Lung adenocarcinoma (LUAD) remains a leading cause of cancer-related deaths worldwide. YTHDF2 is a reader of N6-methyladenosine (m6A) on RNA and plays a critical role in the initiation and propagation of myeloid leukemia; however, whether YTHDF2 controls the development of LUAD remains to be explored. Here, we found that YTHDF2 was significantly upregulated in LUAD compared with paracancerous normal tissues, and YTHDF2 knockdown drastically inhibited, while its overexpression promoted, cell growth, colony formation and migration of LUAD cells in vitro. In addition, YTHDF2 knockdown significantly inhibited tumorigenesis in a murine tumor xenograft model. Through the integrative analysis of RNA-seq, m6A-seq, CLIP-seq, and RIP-seq datasets, we identified a set of potential direct targets of YTHDF2 in LUAD, among which we confirmed AXIN1, which encodes a negative regulator of the Wnt/β-catenin signaling, as a direct target of YTHDF2. YTHDF2 promoted AXIN1 mRNA decay and subsequently activated the Wnt/β-catenin signaling. Knockout of AXIN1 sufficiently rescued the inhibitory effect of YTHDF2 depletion on lung cancer cell proliferation, colony-formation, and migration. These results revealed YTHDF2 to be a contributor of LUAD development acting through the upregulation of the AXIN1/Wnt/β-catenin signaling, which can be a potential therapeutic target for LUAD.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

