Functional and diffusion MRI reveal the neurophysiological basis of neonates' noxious-stimulus evoked brain activity
- PMID: 33980860
- PMCID: PMC8115252
- DOI: 10.1038/s41467-021-22960-0
Functional and diffusion MRI reveal the neurophysiological basis of neonates' noxious-stimulus evoked brain activity
Abstract
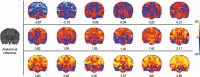
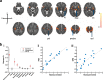
Understanding the neurophysiology underlying neonatal responses to noxious stimulation is central to improving early life pain management. In this neonatal multimodal MRI study, we use resting-state and diffusion MRI to investigate inter-individual variability in noxious-stimulus evoked brain activity. We observe that cerebral haemodynamic responses to experimental noxious stimulation can be predicted from separately acquired resting-state brain activity (n = 18). Applying this prediction model to independent Developing Human Connectome Project data (n = 215), we identify negative associations between predicted noxious-stimulus evoked responses and white matter mean diffusivity. These associations are subsequently confirmed in the original noxious stimulation paradigm dataset, validating the prediction model. Here, we observe that noxious-stimulus evoked brain activity in healthy neonates is coupled to resting-state activity and white matter microstructure, that neural features can be used to predict responses to noxious stimulation, and that the dHCP dataset could be utilised for future exploratory research of early life pain system neurophysiology.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Lee, G. & Stevens, B. in Oxford Textbook of Paediatric Pain 353–369 (Oxford University Press, 2013).
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

