Non-invasive, opsin-free mid-infrared modulation activates cortical neurons and accelerates associative learning
- PMID: 33980868
- PMCID: PMC8115038
- DOI: 10.1038/s41467-021-23025-y
Non-invasive, opsin-free mid-infrared modulation activates cortical neurons and accelerates associative learning
Abstract
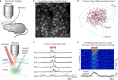
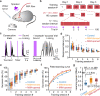
Neurostimulant drugs or magnetic/electrical stimulation techniques can overcome attention deficits, but these drugs or techniques are weakly beneficial in boosting the learning capabilities of healthy subjects. Here, we report a stimulation technique, mid-infrared modulation (MIM), that delivers mid-infrared light energy through the opened skull or even non-invasively through a thinned intact skull and can activate brain neurons in vivo without introducing any exogeneous gene. Using c-Fos immunohistochemistry, in vivo single-cell electrophysiology and two-photon Ca2+ imaging in mice, we demonstrate that MIM significantly induces firing activities of neurons in the targeted cortical area. Moreover, mice that receive MIM targeting to the auditory cortex during an auditory associative learning task exhibit a faster learning speed (~50% faster) than control mice. Together, this non-invasive, opsin-free MIM technique is demonstrated with potential for modulating neuronal activity.
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Task-dependent and cell-type-specific Fos enhancement in rat sensory cortices during audio-visual discrimination.Eur J Neurosci. 2002 Feb;15(4):735-43. doi: 10.1046/j.1460-9568.2002.01905.x. Eur J Neurosci. 2002. PMID: 11886453
-
Perception and recognition discriminated in the mouse auditory cortex by c-Fos labeling.Neuroreport. 1999 Aug 2;10(11):2341-5. doi: 10.1097/00001756-199908020-00022. Neuroreport. 1999. PMID: 10439460
-
Fos imaging reveals differential neuronal activation of areas of rat temporal cortex by novel and familiar sounds.Eur J Neurosci. 2001 Jul;14(1):118-24. doi: 10.1046/j.0953-816x.2001.01625.x. Eur J Neurosci. 2001. PMID: 11488955
-
Comparison of frequency-specific c-Fos expression and fluoro-2-deoxyglucose uptake in auditory cortex of gerbils (Meriones unguiculatus).Eur J Neurosci. 1995 Jul 1;7(7):1614-26. doi: 10.1111/j.1460-9568.1995.tb01157.x. Eur J Neurosci. 1995. PMID: 7551188
-
Statistic parametric mapping of changes in gene activity in animal brain during acoustic stimulation.Bull Exp Biol Med. 2013 Mar;154(5):697-9. doi: 10.1007/s10517-013-2032-5. Bull Exp Biol Med. 2013. PMID: 23658901
Cited by
-
Study on the Effect of the Envelope of Terahertz Unipolar Stimulation on Cell Membrane Communication-Related Variables.Research (Wash D C). 2025 Jul 15;8:0755. doi: 10.34133/research.0755. eCollection 2025. Research (Wash D C). 2025. PMID: 40666829 Free PMC article.
-
Membrane-mediated modulation of mitochondrial physiology by terahertz waves.Biomed Opt Express. 2024 Jun 3;15(7):4065-4080. doi: 10.1364/BOE.528706. eCollection 2024 Jul 1. Biomed Opt Express. 2024. PMID: 39022554 Free PMC article.
-
Terahertz Photons Improve Cognitive Functions in Posttraumatic Stress Disorder.Research (Wash D C). 2023 Dec 15;6:0278. doi: 10.34133/research.0278. eCollection 2023. Research (Wash D C). 2023. PMID: 38111677 Free PMC article.
-
Structural Insights and Influence of Terahertz Waves in Midinfrared Region on Kv1.2 Channel Selectivity Filter.ACS Omega. 2024 Feb 12;9(8):9702-9713. doi: 10.1021/acsomega.3c09801. eCollection 2024 Feb 27. ACS Omega. 2024. PMID: 38434859 Free PMC article.
-
Dielectric dispersion characteristics of the phospholipid bilayer with subnanometer resolution from terahertz to mid-infrared.Front Bioeng Biotechnol. 2022 Aug 31;10:984880. doi: 10.3389/fbioe.2022.984880. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 36118579 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

