Physiological and transcriptomic analyses characterized high temperature stress response mechanisms in Sorbus pohuashanensis
- PMID: 33980903
- PMCID: PMC8115228
- DOI: 10.1038/s41598-021-89418-7
Physiological and transcriptomic analyses characterized high temperature stress response mechanisms in Sorbus pohuashanensis
Abstract
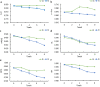
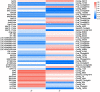
Sorbus pohuashanensis (Hance) Hedl. is a Chinese native alpine tree species, but the problem of introducing S. pohuashanensis to low altitude areas has not been solved. In this study, we aimed to explore the molecular regulatory network of S. pohuashanensis in response to high-temperature stress using RNA-Sequencing technology and physiological and biochemical determination. Based on transcriptomic data, we obtained 1221 genes (752 up-regulated and 469 down-regulated) that were differentially expressed during 8 h 43℃ treatment and candidate genes were related to calcium signaling pathway, plant hormone signal transduction, heat shock factors, chaperones, ubiquitin mediated proteolysis, cell wall modification, ROS scavenging enzymes, detoxification and energy metabolism. The analysis of high temperature response at the physiological level and biochemical level were performed. The chlorophyll fluorescence parameters of leaf cells decreased, the content of osmotic regulators increased, and the activity of ROS scavenging enzymes decreased. The molecular regulatory network of S. pohuashanensis in response to high-temperature stress was preliminarily revealed in this study, which provides fundamental information improving introducing methods and discovering heat-tolerant genes involved in high-temperature stress in this species and provides a reference for other plants of the genus Sorbus.
Conflict of interest statement
The authors declare no competing interests.
Figures










References
-
- Abbo S, Lev-Yadun S, Gopher A. Plant domestication and crop evolution in the Near East: on events and processes. Crit. Rev. Plant Sci. 2012;31:241–257. doi: 10.1080/07352689.2011.645428. - DOI
-
- Castro AVP, Brito P, González-Rodríguez A. Changes in thermic limits and acclimation assessment for an alpine plant by chlorophyll fluorescence analysis: Fv/Fm vs Rfd. Photosynthetica. 2017;56:527–536. doi: 10.1007/s11099-017-0691-6. - DOI
-
- Mani MS. Alpine plants Book reviews: ecology and phytogeography of high altitude plants of the Northwest Himalaya Introduction to high altitude botany. Science. 1979;206:883–896.
-
- Berry JA, Bjorkman O. Photosynthetic response and adaptation to temperature in higher plants. Annu. Rev. Plant Physiol. 2003;31:491–543. doi: 10.1146/annurev.pp.31.060180.002423. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

