Paracetamol (acetaminophen) rescues cognitive decline, neuroinflammation and cytoskeletal alterations in a model of post-operative cognitive decline (POCD) in middle-aged rats
- PMID: 33980934
- PMCID: PMC8115335
- DOI: 10.1038/s41598-021-89629-y
Paracetamol (acetaminophen) rescues cognitive decline, neuroinflammation and cytoskeletal alterations in a model of post-operative cognitive decline (POCD) in middle-aged rats
Abstract
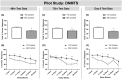
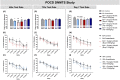
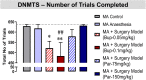
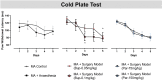
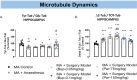
Post-operative cognitive dysfunction (POCD) is a debilitating clinical phenomenon in elderly patients. Management of pain in elderly is complicated because analgesic opiates elicit major side effects. In contrast, paracetamol (acetaminophen) has shown analgesic efficacy, no impact on cognition, and its side effects are well tolerated. We investigated the efficacy of paracetamol, compared to the opioid analgesic buprenorphine, in a model of POCD by investigating cognitive decline, allodynia, peripheral and hippocampal cytokines levels, and hippocampal microtubule dynamics as a key modulator of synaptic plasticity. A POCD model was developed in middle-aged (MA) rats by inducing a tibia fracture via orthopaedic surgery. Control MA rats did not undergo any surgery and only received isoflurane anaesthesia. We demonstrated that cognitive decline and increased allodynia following surgery was prevented in paracetamol-treated animals, but not in animals which were exposed to anesthesia alone or underwent the surgery and received buprenorphine. Behavioral alterations were associated with different peripheral cytokine changes between buprenorphine and paracetamol treated animals. Buprenorphine showed no central effects, while paracetamol showed modulatory effects on hippocampal cytokines and markers of microtubule dynamics which were suggestive of neuroprotection. Our data provide the first experimental evidence corroborating the use of paracetamol as first-choice analgesic in POCD.
Conflict of interest statement
M.B. work has been funded by Angelini Pharma S.p.A. B.G., L.D., C.M. and F.P.D.G. are employees of Angelini Pharma S.p.A. C.C. is an employee of Ulysses Neuroscience Ltd. M.B. owns Ulysses Neuroscience Ltd. and he was an employee of Transpharmation Ltd. J.P. and E.S. are employees of Transpharmation Ltd.
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

