Molecular docking analysis and evaluation of the antibacterial and antioxidant activities of the constituents of Ocimum cufodontii
- PMID: 33980935
- PMCID: PMC8115310
- DOI: 10.1038/s41598-021-89557-x
Molecular docking analysis and evaluation of the antibacterial and antioxidant activities of the constituents of Ocimum cufodontii
Abstract
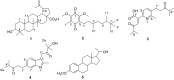
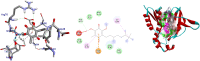
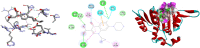
Ocimum cufodontii ((Lanza) A.J.Paton) has been traditionally used in Ethiopia against bacteria. The extracts of the leaves and roots of O. cufodontii after silica gel column chromatography furnished compounds 1-5, compounds 3 and 4 are new natural products. The oil from the hydro-distillation of the leaves, after analyzed with GC-MS, has led to the identification of β-caryophyllene as a principal component, suggesting the essential oil as medicine and spices to enhance the taste of food. The constituents of O. cufodontii were assessed for their antibacterial activity against E. coli, K. pneumonia, S. typhymurium and S. aureus. The best activity was displayed against S. aureus by the hexane extract of the roots, compound 4, and the essential oil with an inhibition zone of 17, 15, and 19 mm, respectively. Molecular docking analysis revealed that compound 1 has better docking efficiency and forms hydrophobic interactions with five amino acids (ARG192, PHE196, GLU185, GLU193, and LYS189). This suggests that the compounds may act as potential inhibitors of DNA gyrase. The constituents were also assessed for their antioxidant activities using DPPH, ferric thicyanate and ferric reducing power assay. The hexane extracts of the roots inhibited the DPPH radical and peroxide formation by 90.5 and 83%, respectively, suggesting the potential of the extract as an antioxidant. Furthermore, the hexane extract of the roots of O. cufodontii exhibited the maximum reducing power compared with the EtOAc and methanol extracts. Hence, the activity displayed herein indicated as the plant has great potential as a remedy for diseases caused by bacteria and radicals.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Makri O, Kintzios S. Ocimum sp. (Basil): Botany, Cultivation, pharmaceutical properties, and biotechnology. J. Herbs Spices Med. Plants. 2007;13(3):1.
-
- Roberto F, James ES. Chemical characterization of basil (Ocimum Spp.) found in the markets and used in traditional medicine in Brazil. Econ. Bot. 2000;54(2):207–216. doi: 10.1007/BF02907824. - DOI
-
- Simon, J. E., Quinn, J., Murray, R. G. Basil: A source of essential oils. Adv. New Crops Res. 484–489 (1990).
-
- Kadhim MJ, Sosa AA, Hameed LH. Evaluation of anti-bacterial activity and bioactive chemical analysis of Ocimum basilicum using Fourier transform infrared (FT-IR) and gas chromatographymass spectrometry (GC-MS) techniques. J. Pharmacogn. Phytother. 2016;8(6):127–146. doi: 10.5897/JPP2015.0366. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

